Cholesterol taste avoidance in Drosophila melanogaster
- PMID: 40244888
- PMCID: PMC12005718
- DOI: 10.7554/eLife.106256
Cholesterol taste avoidance in Drosophila melanogaster
Abstract
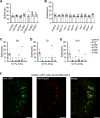
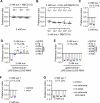
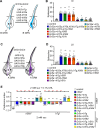
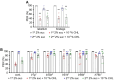
The question as to whether animals taste cholesterol taste is not resolved. This study investigates whether the fruit fly, Drosophila melanogaster, is capable of detecting cholesterol through their gustatory system. We found that flies are indifferent to low levels of cholesterol and avoid higher levels. The avoidance is mediated by gustatory receptor neurons (GRNs), demonstrating that flies can taste cholesterol. The cholesterol-responsive GRNs comprise a subset that also responds to bitter substances. Cholesterol detection depends on five ionotropic receptor (IR) family members, and disrupting any of these genes impairs the flies' ability to avoid cholesterol. Ectopic expressions of these IRs in GRNs reveals two classes of cholesterol receptors, each with three shared IRs and one unique subunit. Additionally, expressing cholesterol receptors in sugar-responsive GRNs confers attraction to cholesterol. This study reveals that flies can taste cholesterol, and that the detection depends on IRs in GRNs.
Keywords: Cholesterol; D. melanogaster; IR51b; IR56d; IR7g; gustatory receptor neurons; neuroscience; taste.
© 2025, Nhuchhen Pradhan et al.
Conflict of interest statement
RN, CM, YL No competing interests declared
Figures




Update of
- doi: 10.1101/2025.02.20.639355
- doi: 10.7554/eLife.106256.1
Similar articles
-
A subset of sweet-sensing neurons identified by IR56d are necessary and sufficient for fatty acid taste.PLoS Genet. 2017 Nov 9;13(11):e1007059. doi: 10.1371/journal.pgen.1007059. eCollection 2017 Nov. PLoS Genet. 2017. PMID: 29121639 Free PMC article.
-
Molecular Basis of Hexanoic Acid Taste in Drosophila melanogaster.Mol Cells. 2023 Jul 31;46(7):451-460. doi: 10.14348/molcells.2023.0035. Epub 2023 May 19. Mol Cells. 2023. PMID: 37202372 Free PMC article.
-
Molecular basis of fatty acid taste in Drosophila.Elife. 2017 Dec 12;6:e30115. doi: 10.7554/eLife.30115. Elife. 2017. PMID: 29231818 Free PMC article.
-
Gustatory Processing in Drosophila melanogaster.Annu Rev Entomol. 2018 Jan 7;63:15-30. doi: 10.1146/annurev-ento-020117-043331. Annu Rev Entomol. 2018. PMID: 29324046 Review.
-
Taste and pheromone perception in the fruit fly Drosophila melanogaster.Pflugers Arch. 2007 Aug;454(5):735-47. doi: 10.1007/s00424-007-0246-y. Epub 2007 May 1. Pflugers Arch. 2007. PMID: 17473934 Review.
Cited by
-
Behavior choices amongst grooming, feeding, and courting in Drosophila show contextual flexibility, not an absolute hierarchy of needs.bioRxiv [Preprint]. 2025 May 13:2025.05.09.653186. doi: 10.1101/2025.05.09.653186. bioRxiv. 2025. PMID: 40463182 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

