The Rise of Chalcohalide Solar Cells: Comprehensive Insights From Materials to Devices
- PMID: 40244923
- PMCID: PMC12097054
- DOI: 10.1002/advs.202413131
The Rise of Chalcohalide Solar Cells: Comprehensive Insights From Materials to Devices
Abstract
While lead-halide perovskites achieve high efficiencies, their toxicity and instability drive the search for safer materials. Chalcohalides, combining chalcogen and halogen anions in versatile structures, emerge as earth-abundant, nontoxic alternatives for efficient photovoltaic (PV) devices. A wide variety of chalcohalide materials, including pnictogen metals-, post-transition metals-, mixed-metals- and organic-inorganic metals-based chalcohalides, offer diverse structural, compositional, and optoelectronic characteristics. Some of these materials have already been experimentally synthesized and integrated into PV devices, achieving efficiencies of 4-6%, while others remain theoretically predicated. Despite these advancements, significant challenges must be addressed to fully realize the potential of chalcohalides as next-generation PV absorbers. This review provides a comprehensive insight of the fundamental properties of chalcohalide materials, emphasizing their unique structures, highly interesting optoelectronic and dielectric properties, to fuel further research and guide the development of high-efficiency chalcohalide solar cells. Various synthesis techniques are discussed, highlighting important and potentially overlooked strategies for fabricating complex quaternary and pentanary chalcohalide materials. Additionally, the working principles of different device structures and recent advances in fabricating efficient chalcohalide solar cells are covered. We hope that this review inspires further exciting research, innovative approaches, and breakthroughs in the field of chalcohalide materials.
Keywords: metal chalcohalides; perovskite‐inspired semiconductors; solar absorber; thin film photovoltaics.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


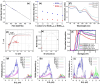


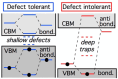
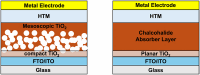






Similar articles
-
Emerging Chalcohalide Materials for Energy Applications.Chem Rev. 2023 Jan 11;123(1):327-378. doi: 10.1021/acs.chemrev.2c00422. Epub 2022 Nov 21. Chem Rev. 2023. PMID: 36410039 Free PMC article. Review.
-
Chalcogenide Perovskites and Perovskite-Based Chalcohalide as Photoabsorbers: A Study of Their Properties, and Potential Photovoltaic Applications.Materials (Basel). 2021 Dec 18;14(24):7857. doi: 10.3390/ma14247857. Materials (Basel). 2021. PMID: 34947450 Free PMC article. Review.
-
Hidden spontaneous polarisation in the chalcohalide photovoltaic absorber Sn2SbS2I3.Mater Horiz. 2021 Oct 4;8(10):2709-2716. doi: 10.1039/d1mh00764e. Mater Horiz. 2021. PMID: 34617541 Free PMC article.
-
Solution processed bismuth oxyiodide (BiOI) thin films and solar cells.Nanotechnology. 2023 May 12;34(30). doi: 10.1088/1361-6528/acc1df. Nanotechnology. 2023. PMID: 37171099
-
Lead-Free Semiconductors: Phase-Evolution and Superior Stability of Multinary Tin Chalcohalides.Chem Mater. 2024 Apr 19;36(9):4542-4552. doi: 10.1021/acs.chemmater.4c00209. eCollection 2024 May 14. Chem Mater. 2024. PMID: 38764751 Free PMC article.
References
-
- a) Jeon N. J., Noh J. H., Kim Y. C., Yang W. S., Ryu S., Seok S. I., Nat. Mater. 2014, 13, 897; - PubMed
- b) Hao F., Stoumpos C. C., Cao D. H., Chang R. P., Kanatzidis M. G., Nat. Photonics 2014, 8, 489;
- c) Saparov B., Mitzi D. B., Chem. Rev. 2016, 116, 4558; - PubMed
- d) Correa‐Baena J.‐P., Saliba M., Buonassisi T., Grätzel M., Abate A., Tress W., Hagfeldt A., Science 2017, 358, 739; - PubMed
- e) Han T. H., Tan S., Xue J., Meng L., Lee J. W., Yang Y., Adv. Mater. 2019, 31, 1803515; - PubMed
- f) Xiao Z., Song Z., Yan Y., Adv. Mater. 2019, 31, 1803792; - PubMed
- g) Tong Y., Najar A., Wang L., Liu L., Du M., Yang J., Li J., Wang K., Liu S., Adv. Sci. 2022, 9, 2105085. - PMC - PubMed
-
- a) Woo J., Christian A. H., Burgess S. A., Jiang Y., Mansoor U. F., Levin M. D., Science 2022, 376, 527; - PMC - PubMed
- b) Liu C., Yang Y., Chen H., Xu J., Liu A., Bati A. S., Zhu H., Grater L., Hadke S. S., Huang C., Science 2023, 382, 810; - PubMed
- c) Chen H., Liu C., Xu J., Maxwell A., Zhou W., Yang Y., Zhou Q., Bati A. S., Wan H., Wang Z., Science 2024, 384, 189. - PubMed
-
- a) National Renewable Energy La boratory (NREL), Interactive Best Research‐Cell Efficiency Chart, https://www.nrel.gov/pv/interactive‐cell‐efficiency.html (accessed: March 2025);
- b) Shi Y., Berry J. J., Zhang F., ACS Energy Lett. 2024, 9, 1305;
- c) Liu S., Lu Y., Yu C., Li J., Luo R., Guo R., Liang H., Jia X., Guo X., Wang Y.‐D., Nature 2024, 628, 306; - PubMed
- d) Lim J., Park N.‐G., Il Seok S., Saliba M., Energy Environ. Sci. 2024, 17, 4390. - PMC - PubMed
-
- a) Seok S. I., Grätzel M., Park N. G., Small 2018, 14, 1704177; - PubMed
- b) Liu J., Zheng D., Wang K., Li Z., Liu S., Peng L., Yang D., Joule 2024, 8, 944;
- c) Park N.‐G., Zhu K., Nat. Rev. Mater. 2020, 5, 333;
- d) Li Z., Klein T. R., Kim D. H., Yang M., Berry J. J., Van Hest M. F., Zhu K., Nat. Rev. Mater. 2018, 3, 18017;
- e) Zhu P., Chen C., Dai J., Zhang Y., Mao R., Chen S., Huang J., Zhu J., Adv. Mater. 2024, 36, 2307357. - PubMed
-
- a) Kim H.‐S., Seo J.‐Y., Park N.‐G., ChemSusChem 2016, 9, 2528; - PubMed
- b) Wang D., Wright M., Elumalai N. K., Uddin A., Sol. Energy Mater. Sol. Cells 2016, 147, 255;
- c) Tiep N. H., Ku Z., Fan H. J., Adv. Energy Mater. 2016, 6, 1501420;
- d) Rong Y., Liu L., Mei A., Li X., Han H., Adv. Energy Mater. 2015, 5, 1501066;
- e) Ono L. K., Qi Y., Liu S., Joule 2018, 2, 1961;
- f) Zhuang J., Wang J., Yan F., Nano‐Micro Lett. 2023, 15, 84; - PMC - PubMed
- g) Wang Q., Phung N., Di Girolamo D., Vivo P., Abate A., Energy Environ. Sci. 2019, 12, 865.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
