UDP-glucose dehydrogenase variants cause dystroglycanopathy
- PMID: 40245099
- PMCID: PMC12172095
- DOI: 10.1002/acn3.70002
UDP-glucose dehydrogenase variants cause dystroglycanopathy
Abstract
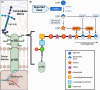
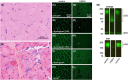
UDP-glucose dehydrogenase (UGDH) variants have been associated with hypotonia, developmental delay, and epilepsy. We report the first pathologic evidence of dystroglycanopathy in siblings with UGDH variants. Both presented around 6 months with developmental delay and elevated creatinine kinase. Sibling A developed epilepsy at age 9 years. Muscle biopsy from sibling A showed necrotizing myopathy with reduced matriglycan immunostaining. Western blot revealed α-dystroglycan with abnormally low molecular weight. The siblings shared pathogenic UGDH variants in trans: c.305G>A p.(R102Q) is predicted to disrupt protein structure and function; c.265-6C>G is deleterious to splicing. We propose that UGDH is an additional dystroglycanopathy gene.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
KDM receives research funding from the Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant (NIH U54 NS053672), Friedreich Ataxia Research Alliance (FARA), Muscular Dystrophy Association (MDA), and the Centers for Disease Control (U01 DD001248). She serves as an advisory board member for MDA and the FSH Society and is a board member for the FARA. She has served on advisory boards for Sarepta Therapeutics, Edgewise Therapeutics, ML Bio, and Asklepios. She receives or has recently received clinical trial funding from PTC Therapeutics, Sarepta Therapeutics, Pfizer, Reata, Fibrogen, Italfarmaco, Biohaven, Novartis, AMO, Capricor, Asklepios, ML Bio, and Scholar Rock.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

