Minimizing Energy Demand in the Conversion of Levulinic Acid to γ‑Valerolactone via Photothermal Catalysis Using Raney Ni
- PMID: 40245161
- PMCID: PMC12140316
- DOI: 10.1002/advs.202416153
Minimizing Energy Demand in the Conversion of Levulinic Acid to γ‑Valerolactone via Photothermal Catalysis Using Raney Ni
Abstract
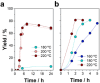
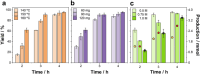
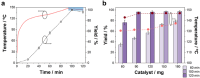
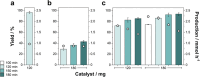
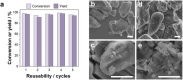
The valorization of lignocellulosic wastes emerges as a prime strategy to mitigate the global carbon footprint. Among the multiple biomass derivatives, γ-valerolactone is particularly attractive as precursor of high-value chemicals, biofuel, green solvent or perfumery. γ-Valerolactone can be synthesized through a hydrogenation reaction from levulinic acid, obtained from cellulose. However, the high energy requirements of this synthetic pathway have hindered its industrial viability. To drastically reduce the reaction energy requirements, here a novel synthetic strategy, based on solvothermal-photothermal processes using cost-effective Raney-Ni as photothermal catalyst, is proposed. First, the use of hydrogen gas is avoided by selecting isopropanol as a safer and greener H-source. Second, a photothermocatalytic process is used to minimize the reaction temperature and time with respect to conventional reactions. This approach exploits the broadband optical absorption of the Raney®-Ni, due to its highly damped plasmonic behavior, to achieve fast and efficient catalyst heating inside the reactor. The photothermal reaction required less than 2 h and just 132 °C to reach over 95% conversion, thereby drastically reducing the reaction time and energy consumption compared to conventional reactions. Importantly, these conditions granted high catalyst reusability. This solvothermal-photothermal approach could offer a sustainable alternative for the industrial production of γ-valerolactone.
Keywords: Raney Ni; biomass valorization; lignocellulosic biomass; photothermo‐catalysis; γ‐valerolactone.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of Kinetic Models for the Catalytic Hydrogenation of Levulinic Acid to γ-Valerolactone over Nickel Catalyst Supported by Titania.Molecules. 2025 Mar 21;30(7):1400. doi: 10.3390/molecules30071400. Molecules. 2025. PMID: 40285868 Free PMC article.
-
Biomass derived efficient conversion of levulinic acid for sustainable production of γ-valerolactone over cobalt based catalyst.J Hazard Mater. 2021 Mar 5;405:123335. doi: 10.1016/j.jhazmat.2020.123335. Epub 2020 Jun 29. J Hazard Mater. 2021. PMID: 33317894
-
The Role of the Hydrogen Source on the Selective Production of γ-Valerolactone and 2-Methyltetrahydrofuran from Levulinic Acid.ChemSusChem. 2016 Sep 8;9(17):2488-95. doi: 10.1002/cssc.201600751. Epub 2016 Aug 2. ChemSusChem. 2016. PMID: 27483194
-
Homogeneous Catalyzed Reactions of Levulinic Acid: To γ-Valerolactone and Beyond.ChemSusChem. 2016 Aug 23;9(16):2037-47. doi: 10.1002/cssc.201600517. Epub 2016 Jul 28. ChemSusChem. 2016. PMID: 27464831 Review.
-
Catalytic valorisation of biomass levulinic acid into gamma valerolactone using formic acid as a H2 donor: a critical review.RSC Adv. 2022 May 6;12(22):13673-13694. doi: 10.1039/d2ra01379g. eCollection 2022 May 5. RSC Adv. 2022. PMID: 35530384 Free PMC article. Review.
References
-
- Wan Azelee N. I., Mahdi H. I., Cheng Y. S., Nordin N., Illias R. M., Rahman R. A., Shaarani S. M., Bhatt P., Yadav S., Chang S. W., Ravindran B., Ashokkumar V., Fuel 2023, 339, 126982.
-
- Kumar B., Bhardwaj N., Agrawal K., Chaturvedi V., Verma P., Fuel Process. Technol. 2020, 199, 106244.
-
- uddeen Safian M. T., Sekeri S. H., Yaqoob A. A., Serrà A., Jamudin M. D., Mohamad Ibrahim M. N., Talanta 2022, 239, 123109. - PubMed
-
- Burhenne L., Messmer J., Aicher T., Laborie M. P., J. Anal. Appl. Pyrolysis 2013, 101, 177.
Grants and funding
LinkOut - more resources
Full Text Sources
