Retinal to Retinal Energy Transfer in a Bistable Microbial Rhodopsin Dimer
- PMID: 40245178
- PMCID: PMC12046560
- DOI: 10.1021/jacs.5c01276
Retinal to Retinal Energy Transfer in a Bistable Microbial Rhodopsin Dimer
Abstract
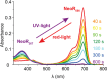
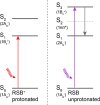
Neorhodopsin (NeoR) is a newly discovered fungal bistable rhodopsin that reversibly photoswitches between UV- and near-IR absorbing states denoted NeoR367 and NeoR690, respectively. NeoR367 represents a deprotonated retinal Schiff base (RSB), while NeoR690 represents a protonated RSB. Cryo-EM studies indicate that NeoR forms homodimers with 29 Å center-to-center distance between the retinal chromophores. UV excitation of NeoR367 takes place to an optically allowed S3 state of 1Bu+ symmetry, which rapidly converts to a low-lying optically forbidden S1 state of 2Ag- symmetry in 39 fs, followed by a multiexponential decay to the ground state on the 1-100 ps time scale. A theoretically predicted nπ* (S2) state does not get populated in any appreciable transient concentration during the excited-state relaxation cascade. We observe an intradimer retinal to retinal excitation energy transfer (EET) process from the NeoR367 S1 state to NeoR690, in competition with photoproduct formation. To quantitatively assess the EET mechanism and rate, we experimentally addressed and modeled the EET process under varying NeoR367-NeoR690 photoequilibrium conditions and determined the EET rate at (200 ps)-1. The NeoR367 S1 state shows a weak stimulated emission band in the near-IR around 700 nm, which may result from mixing with an intramolecular charge-transfer (ICT) state, enhancing the transition dipole moment of the S1-S0 transition and possibly facilitating the EET process. We suggest that EET may bear general relevance to the function of bistable multiwavelength rhodopsin oligomers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Hochbaum D. R.; Zhao Y.; Farhi S. L.; Klapoetke N.; Werley C. A.; Kapoor V.; Zou P.; Kralj J. M.; Maclaurin D.; Smedemark-Margulies N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11 (8), 825–833. 10.1038/nmeth.3000. - DOI - PMC - PubMed
-
- Rozenberg A.; Kaczmarczyk I.; Matzov D.; Vierock J.; Nagata T.; Sugiura M.; Katayama K.; Kawasaki Y.; Konno M.; Nagasaka Y.; et al. Rhodopsin-bestrophin fusion proteins from unicellular algae form gigantic pentameric ion channels. Nat. Struct. Mol. Biol. 2022, 29 (6), 592.10.1038/s41594-022-00783-x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

