Sphingosine-1-Phosphate Receptor 2 Agonist Mobilises Endogenous Muse Cells to Repair Damaged Myocardial Tissue in Male Rabbits
- PMID: 40245180
- PMCID: PMC12005397
- DOI: 10.1111/jcmm.70447
Sphingosine-1-Phosphate Receptor 2 Agonist Mobilises Endogenous Muse Cells to Repair Damaged Myocardial Tissue in Male Rabbits
Abstract
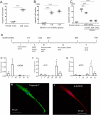
Muse cells, pluripotent stem cells present mainly in the bone marrow (BM) selectively accumulate to damaged tissue by sensing sphingosine-1-phosphate (S1P) and replace damaged cells by differentiating in situ. Acute myocardial infarction (AMI) model rabbits were subcutaneously injected either with Vehicle (n = 15), S1PR2-agonist (n = 16), or S1PR2-agonist + S1PR2-antagonist (n = 10). The number of Muse cells in the peripheral blood was assessed by flow cytometry at 12 h after AMI. The S1PR2-agonist group showed a significant increase in the peripheral-blood Muse cell number at 12 h (p < 0.05), as well as infarct size reduction (p < 0.05) and improvement of left ventricular (LV) function (p < 0.05) at 2 weeks compared with the other 2 groups. The number of peripheral-blood Muse cells positively correlated with LV ejection fraction (p < 0.05) and inversely correlated with infarct size (p < 0.05). Transplanted autologous green fluorescent protein (GFP)-labelled BM-Muse cells into the BM, followed by the administration of either Vehicle (n = 5) or S1PR2 agonist (n = 5) revealed a higher number of homed GFP-Muse cells expressing the cardiac markers troponin-I, α-actinin, connexin-43 and the vascular marker CD31 in the border areas in the S1PR2-agonist group compared with the vehicle group. The mobilisation of endogenous Muse cells using S1PR2-agonist may be a promising therapeutic approach.
Keywords: cardiac function; endogenous muse cells; infarct size; mobilisation; myocardial infarction; sphingosine‐1‐phosphate receptor 2 agonist.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
S1P-S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction.Circ Res. 2018 Apr 13;122(8):1069-1083. doi: 10.1161/CIRCRESAHA.117.311648. Epub 2018 Feb 23. Circ Res. 2018. PMID: 29475983
-
Mobilized Muse Cells After Acute Myocardial Infarction Predict Cardiac Function and Remodeling in the Chronic Phase.Circ J. 2018 Jan 25;82(2):561-571. doi: 10.1253/circj.CJ-17-0552. Epub 2017 Sep 20. Circ J. 2018. PMID: 28931784 Clinical Trial.
-
Human Muse cells reduce myocardial infarct size and improve cardiac function without causing arrythmias in a swine model of acute myocardial infarction.PLoS One. 2022 Mar 24;17(3):e0265347. doi: 10.1371/journal.pone.0265347. eCollection 2022. PLoS One. 2022. PMID: 35324926 Free PMC article.
-
Muse Cells Are Endogenous Reparative Stem Cells.Adv Exp Med Biol. 2018;1103:43-68. doi: 10.1007/978-4-431-56847-6_3. Adv Exp Med Biol. 2018. PMID: 30484223 Review.
-
Acute Myocardial Infarction, Cardioprotection, and Muse Cells.Adv Exp Med Biol. 2018;1103:153-166. doi: 10.1007/978-4-431-56847-6_8. Adv Exp Med Biol. 2018. PMID: 30484228 Review.
Cited by
-
Comparison of MSCs and Muse cells: the possible use for healthspan optimization.Biogerontology. 2025 Jul 2;26(4):139. doi: 10.1007/s10522-025-10275-2. Biogerontology. 2025. PMID: 40601066 Free PMC article.
References
-
- Pfeffer M. A. and Braunwald E., “Ventricular Remodeling After Myocardial Infarction. Experimental Observations and Clinical Implications,” Circulation 81 (1990): 1161–1172. - PubMed
-
- Aso S., Imamura H., Sekiguchi Y., et al., “Incidence and Mortality of Acute Myocardial Infarction. A Population‐Based Study Including Patients With Out‐Of‐Hospital Cardiac Arrest,” International Heart Journal 52 (2011): 197–202. - PubMed
-
- Yeh R. W., Sidney S., Chandra M., Sorel M., Selby J. V., and Go A. S., “Population Trends in the Incidence and Outcomes of Acute Myocardial Infarction,” New England Journal of Medicine 362 (2010): 2155–2165. - PubMed
-
- Chew D. S., Heikki H., Schmidt G., et al., “Change in Left Ventricular Ejection Fraction Following First Myocardial Infarction and Outcome,” JACC Clin Electrophysiol 4 (2018): 672–682. - PubMed
-
- Bolognese L., Neskovic A. N., Parodi G., et al., “Left Ventricular Remodeling After Primary Coronary Angioplasty: Patterns of Left Ventricular Dilation and Long‐Term Prognostic Implications,” Circulation 106 (2002): 2351–2357. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

