Subcutaneous Ocrelizumab in Patients With Multiple Sclerosis: Results of the Phase 3 OCARINA II Study
- PMID: 40245351
- PMCID: PMC12006663
- DOI: 10.1212/WNL.0000000000213574
Subcutaneous Ocrelizumab in Patients With Multiple Sclerosis: Results of the Phase 3 OCARINA II Study
Erratum in
-
Subcutaneous Ocrelizumab in Patients With Multiple Sclerosis: Results of the Phase 3 OCARINA II Study.Neurology. 2025 Aug 12;105(3):e213909. doi: 10.1212/WNL.0000000000213909. Epub 2025 Jul 2. Neurology. 2025. PMID: 40601887 Free PMC article. No abstract available.
Abstract
Background and objectives: IV-administered ocrelizumab (OCR) is approved for the treatment of relapsing and primary progressive multiple sclerosis (RMS/PPMS). OCARINA II (NCT05232825) was designed to demonstrate noninferiority in drug exposure of OCR subcutaneous (SC) vs IV administration.
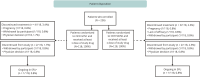
Methods: This phase 3, randomized, open-label study enrolled OCR-naive patients aged 18-65 years with RMS/PPMS and an Expanded Disability Status Scale score of 0-6.5. Patients received OCR IV 600 mg or OCR SC 920 mg (controlled period), followed by OCR SC 920 mg every 24 weeks, up to week 96 (OCR IV/SC and OCR SC/SC). The primary end point was OCR area under the serum concentration-time curve from day 1 to week 12 (AUCW1‒12); other end points included clinical, biomarker, and pharmacodynamic outcomes and safety data.
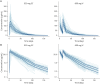
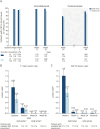
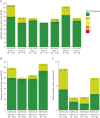
Results: Baseline demographics were balanced across OCR IV/SC and OCR SC/SC arms (N = 118/118, 40.0 ± 11.9/39.9 ± 11.4 years, 59.3%/65.3% female, 89.0%/89.0% with RMS). The study demonstrated noninferiority of OCR SC 920 mg to OCR IV 600 mg for the primary end point AUCW1-12 and also over the dosing interval for AUCW1‒24 (geometric mean ratios [90% CI] 1.29 [1.23-1.35] and 1.27 [1.21-1.34], respectively). At week 48, 111 of 118 (OCR IV/SC) and 114 of 118 (OCR SC/SC) had received OCR SC. A near-complete suppression of MRI activity was reported in OCR IV/SC and OCR SC/SC: 0 of 113 and 0 of 113 patients had T1 lesions while 1 of 114 and 1 of 113 had 2 and 1 new/enlarging T2 lesions, respectively. Two patients (1.9%) in each arm had 1 relapse, and 1 patient (0.9%; OCR SC/SC) had 2 relapses. In both arms, rapid and sustained B-cell depletion was observed and serum neurofilament light chain reduction was comparable. Patients receiving at least 1 dose of OCR SC 920 mg in the OCR IV/SC and OCR SC/SC arms reported adverse events (AEs): 75.4% and 86.4%, and serious AEs: 5.9% and 2.5%. The most frequently reported AEs were injection reactions (IRs, 51.5%); local and systemic IRs were experienced by 117 of 233 patients (50.2%) and 27 of 233 patients (11.6%), respectively. All IRs were mild/moderate; intensity and duration decreased with subsequent injections.
Discussion: The OCR SC formulation demonstrated noninferiority to OCR IV formulation regarding drug exposure, providing comparable efficacy and safety and an additional treatment option for patients with multiple sclerosis.
Classification of evidence: This study provides Class II evidence that a single SC injection of 920 mg of OCR achieves a noninferior 12-week area under serum concentration-time curve to that of 2 IV infusions of 300-mg OCR administered 2 weeks apart.
Trial registration information: ClinicalTrials.gov Identifier NCT05232825; submitted: January 27, 2022; first patient enrolled: May 3, 2022; available at: clinicaltrials.gov/study/NCT05232825?term=NCT05232825&rank=1.
Conflict of interest statement
S.D. Newsome received consultancy fees for scientific advisory boards from Biogen, Genentech Inc., Bristol Myers Squibb, EMD Serono, Greenwich Biosciences, Horizon Therapeutics, Novartis, and TG Therapeutics; is a study lead PI for a Roche clinical trial program; and received research funding (paid directly to institution) from Biogen, Lundbeck, Roche, Genentech Inc., the National MS Society, the Stiff Person Syndrome Research Foundation, the Department of Defense, and the Patient-Centered Outcomes Research Institute. E. Krzystanek received consultancy fees for scientific advisory boards from Biogen, Merck-Serono, Bayer, Roche, Novartis, and the Polish Multiple Sclerosis Society; is a study lead PI for Roche, TG Therapeutics, Merck, Biogen, Lundbeck, and Janssen clinical trial programs; and received compensation for speaking services from Biogen, Bayer, Novartis, UCB, Roche, Merck-Serono, Teva, Lundbeck, Pfizer, Sandoz, and Sanofi-Genzyme. K.W. Selmaj received honoraria for speaking, consulting, and serving for advisory boards for AstraZeneca, Merck, Novartis, Roche, Biogen, Celgene, Bristol Myers Squibb, and TG Therapeutics. M. Dufek received compensation for travel, speaker honoraria, and consultancy fees from Biogen Idec., Novartis, Merck-Serono, Roche, Sanofi-Genzyme, Teva, and Janssen-Cilag. L. Goldstick received consultancy fees from EMD Serono, Bristol Myers Squibb, Biogen, Sanofi-Genzyme, and Roche/Genentech; and has received research support from Biogen, Roche/Genentech, and Sanofi-Genzyme. C. Pozzilli has served on scientific advisory boards and consultancy and/or received speaking fees from Almirall, Alexion Pharmaceutical Inc., Biogen, Janssen, Roche, Merck, and Novartis; and has received research support from Almirall, Biogen, Roche, Merck, and Novartis. C. Figueiredo, B. Townsend, and H. Kletzl are employees of and shareholders in F. Hoffmann-La Roche Ltd. O. Bortolami was a contractor for F. Hoffmann-La Roche Ltd. until December 2023; his current affiliation is IQVIA RDS. D. Zecevic, C. Giacobino, and S. Clinch are employees of and shareholders in F. Hoffmann-La Roche Ltd. Y.-A. Shen is an employee of Genentech Inc., and a shareholder in F. Hoffmann-La Roche Ltd. G.D. Bhullar and H.-M. Schneble are employees of and shareholders in F. Hoffmann-La Roche Ltd. D. Centonze acted as an advisory board member and received honoraria for speaking or consultancy fees from Alexion, Almirall, Amicus, Bayer, Biogen, Bristol Myers Squibb, Celgene, Chiesi, GW Pharmaceuticals, Horizon, Janssen, Lundbeck, Merck-Serono, Novartis, Roche, Sandoz, Sanofi-Genzyme, Viatris, and Teva; and is the PI in clinical trials of Biogen, Bristol Myers Squibb, Merck-Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Actelion. Go to
Figures

References
-
- OCREVUS (ocrelizumab). Prescribing Information. Genentech, Inc., South San Francisco, CA. Updated June 2024; Accessed March 25, 2025. gene.com/download/pdf/ocrevus_prescribing.pdf.
-
- OCREVUS (ocrelizumab). Summary of Product Characteristics. Roche Pharma AG, Grenzach-Wyhlen, Germany. Updated March 2025. Accessed March 25, 2025. ema.europa.eu/en/documents/product-information/ocrevus-epar-product-info....
-
- F. Hoffmann-La Roche Ltd. Data on file. 2024. OCREVUS (Ocrelizumab). Cumulative MULTIPLE SCLEROSIS patient exposure.
-
- Filippi M, Grimaldi L, Conte A, et al. ; EASIER Study Working Group. Intravenous or subcutaneous natalizumab in patients with relapsing-remitting multiple sclerosis: investigation on efficiency and savings-the EASIER study. J Neurol. 2024;271(1):340-354. doi: 10.1007/s00415-023-11955-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
