An unconventional T cell nexus drives HCK-mediated chronic obstructive pulmonary disease in mice
- PMID: 40245497
- PMCID: PMC12143211
- DOI: 10.1016/j.ebiom.2025.105707
An unconventional T cell nexus drives HCK-mediated chronic obstructive pulmonary disease in mice
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a heterogeneous inflammatory lung disease leading to progressive, destructive lung function decline, disability and death, and it is refractory to all current treatments. Haematopoietic cell kinase (HCK) is a druggable SRC-family non-receptor protein tyrosine kinase and COPD candidate gene. It is implicated in the chronic and non-resolving inflammation that causes mucosecretory bronchitis and destruction of small airways and alveoli, but how it drives pathophysiology remains obscure.
Methods: Studies primarily utilised gene-targeted mice with a gain-of-function mutation in Hck that rendered the enzyme constitutively active. Bone marrow chimeras were established to determine the origin of disease, and the lung disease was investigated using histopathology, morphometry, flow cytometry and single-cell sequencing techniques. Detailed pathways mediating disease pathogenesis were examined using specialised knockout mice.
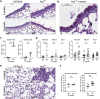
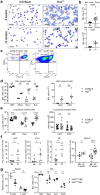
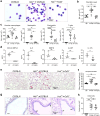
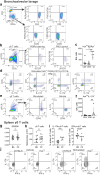
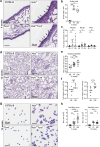
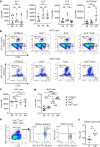
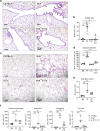
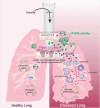
Findings: HckF/F mice developed intense granulocytic mucosecretory inflammation. Bone marrow chimeras revealed that stromal-derived granulocyte-colony-stimulating factor (G-CSF) resulted in lung inflammation and emphysema but not mucus production; while its upstream regulator, interleukin (IL)-17A, itself implicated in emphysema and mucus overproduction, was produced by Vγ6Vδ1 T cells that were recruited to airspaces. Nonetheless, lung disease was unchanged upon genetic deletion of γδ T cells, due to niche-filling expansion of IL-17A-producing mucosal-associated invariant T cells. Strikingly, IL-17A deletion abrogated inflammation, alveolar destruction and mucus overproduction in HckF/F lungs.
Interpretation: These findings highlight the role of HCK as an apical regulator of an unconventional T cell axis that drives IL-17A/G-CSF/granulocyte-mediated pathology in COPD, and underscore the rationale for therapeutically targeting HCK.
Funding: This work received support from the National Health and Medical Research Council Australia, the Victorian Cancer Agency, Melbourne Australia, the Australian Research Council, the Australian Government and the School of Translational Medicine, Monash University, Australia.
Keywords: Chronic obstructive pulmonary disease; Constitutive HCK activation; Granulocytes; IL-17A/G-CSF axis; Mucus-producing goblet cells; Unconventional T cells.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests ATH and TAG received funding to attend the Australian and New Zealand Society of Immunology conference in 2022 and 2023 respectively; GPA received funding from RAGE Biotechnology, acted as a consultant for RAGE Biotechnology, ENA Respiratory and Pieris Pharmaceuticals, received honoraria from AstraZeneca, GSK and Sanofi, support from Griffith University and Thoracic Society of Australia and New Zealand (TSANZ), participated on Data Safety Monitoring Boards for PACE and INHERIT studies, was a Board Member of TSANZ, and received stock options from ENA Respiratory; and, MLH received grant funding from Lupus Research Alliance and RAGE Biotechnology. The remaining authors declare that they have no competing interests.
Figures


Similar articles
-
HIV-1 Nef Induces Hck/Lyn-Dependent Expansion of Myeloid-Derived Suppressor Cells Associated with Elevated Interleukin-17/G-CSF Levels.J Virol. 2021 Aug 10;95(17):e0047121. doi: 10.1128/JVI.00471-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34106001 Free PMC article.
-
γδ+ T-cell-derived IL-17A stimulates airway epithelial/stromal cells to secrete G-CSF, promoting lung-specific pathogenic Siglec-F+ neutrophil development in PPE-induced emphysema.Cell Mol Immunol. 2025 Jul;22(7):791-805. doi: 10.1038/s41423-025-01301-x. Epub 2025 Jun 3. Cell Mol Immunol. 2025. PMID: 40461699 Free PMC article.
-
NR1D1 mitigates IL-17a-induced small airway remodeling in biomass smoke-induced COPD.Toxicol Lett. 2025 Jun;409:74-86. doi: 10.1016/j.toxlet.2025.05.002. Epub 2025 May 7. Toxicol Lett. 2025. PMID: 40345267
-
Inflammatory cells and chronic obstructive pulmonary disease.Curr Drug Targets Inflamm Allergy. 2005 Dec;4(6):607-18. doi: 10.2174/156801005774912824. Curr Drug Targets Inflamm Allergy. 2005. PMID: 17305517 Review.
-
The Influence of Innate Lymphoid Cells and Unconventional T Cells in Chronic Inflammatory Lung Disease.Front Immunol. 2019 Jul 11;10:1597. doi: 10.3389/fimmu.2019.01597. eCollection 2019. Front Immunol. 2019. PMID: 31354734 Free PMC article. Review.
Cited by
-
HCKing COPD: unveiling the role of HCK in COPD pathogenesis.EBioMedicine. 2025 May;115:105714. doi: 10.1016/j.ebiom.2025.105714. Epub 2025 Apr 17. EBioMedicine. 2025. PMID: 40245496 Free PMC article. No abstract available.
References
-
- Vestbo J., Prescott E., Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153(5):1530–1535. - PubMed
-
- Takeyama K., Agustí C., Ueki I., Lausier J., Cardell L.O., Nadel J.A. Neutrophil-dependent goblet cell degranulation: role of membrane-bound elastase and adhesion molecules. Am J Physiol. 1998;275(2):L294–L302. - PubMed
-
- Fischer B.M., Voynow J.A. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26(4):447–452. - PubMed
-
- Voynow J.A., Fischer B.M., Malarkey D.E., et al. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1293–L1302. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

