Risk Stratification for Trastuzumab-Induced Cardiac Dysfunction and Potential Implications for Surveillance
- PMID: 40246379
- PMCID: PMC12046757
- DOI: 10.1016/j.jaccao.2024.12.007
Risk Stratification for Trastuzumab-Induced Cardiac Dysfunction and Potential Implications for Surveillance
Abstract
Background: Although patient factors and sequential anthracycline use contribute to risk for cancer therapy-related cardiac dysfunction (CTRCD) with HER2-directed cancer therapy, frequent (every 3 months) left ventricular ejection fraction (LVEF) surveillance is recommended irrespective of baseline risk.
Objectives: The aim of this study was to examine the incidence of trastuzumab-associated CTRCD in a contemporary cohort with HER2-positive breast cancer and assess the performance of a risk assessment tool to identify patients at low risk for CTRCD to guide risk-based surveillance strategies.
Methods: A retrospective cohort of patients with HER2-positive breast cancer treated with trastuzumab at a tertiary cancer center was examined. Patients were categorized as low, medium, and high or very high risk for CTRCD by Heart Failure Association/International Cardio-Oncology Society risk assessment.
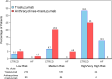
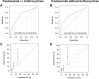
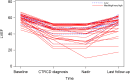
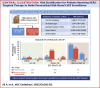
Results: Of 496 patients treated with trastuzumab, 29.8% also received anthracyclines. Over a median follow-up period of 51 months, 8.7% developed CTRCD, but only 1.6% had associated heart failure (HF). CTRCD rates were 3.6%, 12.8%, and 32.1% in low-risk, medium-risk, and high or very high risk groups, respectively. HF incidence was 0.4% in the low-risk group and 2.1% in the medium-risk group, with no HF in patients at low- or medium-risk who received trastuzumab without anthracyclines. HF was observed in 11% of high-risk patients. The risk assessment had a negative predictive value for CTRCD in low vs moderate- or high-risk patients of 96.4% (95% CI: 93.5%-98.3%).
Conclusions: The findings support the exploration of a prospective personalized risk-based approach to cardiac LVEF surveillance during trastuzumab therapy. Less frequent LVEF monitoring in low-risk patients may optimize resource use and reduce patient burden without compromising safety.
Keywords: HER2-targeted therapy; anthracycline; breast cancer; cancer therapy–related cardiac dysfunction; cardiomyopathy; echocardiography; heart failure; imaging; risk prediction; screening; surveillance; trastuzumab.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures The statistical analysis work was supported in part by the Cancer Center Support Grant (National Cancer Institute grant P30 CA016672). Dr Deswal is supported in part by the Ting Tsung and Wei Fong Chao distinguished chair. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
-
- Romond E.H., Perez E.A., Bryant J., et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

