Characterizing the Diversity of Layer 2/3 Human Neocortical Neurons in Pediatric Epilepsy
- PMID: 40246555
- PMCID: PMC12061357
- DOI: 10.1523/ENEURO.0247-24.2025
Characterizing the Diversity of Layer 2/3 Human Neocortical Neurons in Pediatric Epilepsy
Abstract
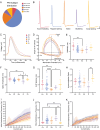
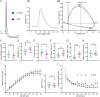
Childhood epilepsy is a common and devastating condition, for which many children still do not have adequate treatment. Some children with drug-resistant epilepsy require surgical excision of epileptogenic brain tissue for seizure control, affording the opportunity to study this tissue ex vivo to interrogate human epileptic neurons for potentially hyperexcitable perturbations in intrinsic electrophysiological properties. In this study, we characterized the diversity of layer L2/3 (L2/3) pyramidal neurons (PNs) in ex vivo brain slices from pediatric patients with epilepsy. We found a remarkable diversity in the firing properties of epileptic L2/3 PNs: five distinct subpopulations were identified. Additionally, we investigated whether the etiology of epilepsy influenced the intrinsic neuronal properties of L2/3 PNs when comparing tissue from patients with epilepsy due to malformations of cortical development (MCDs), other forms of epilepsy (OEs), or with deep-seated tumors. When comparing epileptic with control L2/3 PNs, we observed a decrease in voltage sag and lower maximum firing rates. Moreover, we found that MCD and OE L2/3 PNs were mostly similar indicating that epilepsy etiology may not outweigh the influences of epileptiform activity on L2/3 PN physiology. Lastly, we show that the proconvulsant drug, 4-aminopyridine (4-AP), leads to increased AP half-width, reduced firing rate accommodation, and slower AHPs. These changes imply that 4-AP induces an increase in [K+]o and a resultant increase in AP duration, leading to the release of more excitatory neurotransmitters per action potential, thereby promoting network hyperexcitability.
Keywords: DNET; focal cortical dysplasia; gliosis; intrinsic properties; layer 2/3 pyramidal neurons; tuberous sclerosis.
Copyright © 2025 Kushner et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
