CD22-targeted chimeric antigen receptor-modified T cells for children and adults with relapse of B-cell acute lymphoblastic leukemia after CD19-directed immunotherapy
- PMID: 40246579
- PMCID: PMC12007026
- DOI: 10.1136/jitc-2025-011549
CD22-targeted chimeric antigen receptor-modified T cells for children and adults with relapse of B-cell acute lymphoblastic leukemia after CD19-directed immunotherapy
Abstract
Background: Relapse of B-cell acute lymphoblastic leukemia (B-ALL) with CD19-antigen loss after CD19-targeted chimeric antigen receptor (CAR) T-cell therapy has a dismal prognosis. Novel immunotherapeutic strategies for this patient population are urgently needed.
Methods: We tested a novel, fully human anti-CD22/4-1BB CAR T-cell construct, CART22-65s, in parallel phase I studies for pediatric and adult B-ALL. After lymphodepletion, CART22-65s was infused using a 3-day fractionated dosing scheme, allowing for omission of the second and third doses in cases of early cytokine release syndrome (CRS).
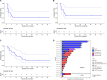
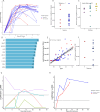
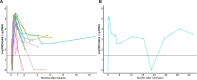
Results: Twenty-two patients, all with relapse after prior CD19-directed immunotherapy, were enrolled. Of 19 infused patients (pediatric, n=17; adult, n=2), 14 (74%) achieved a complete remission (CR), including 4 of 6 (67%) patients refractory to prior inotuzumab. Five of 14 patients in a CR proceeded to consolidative hematopoietic cell transplantation (HCT). With a median follow-up of 38 months, the 12-month relapse-free survival rate was 38.4% (95% CI 19.3% to 76.5%) and overall survival rate was 52.6% (95% CI 34.3% to 80.6%). Two patients received additional CART22-65s treatments for subsequent CD22-positive relapses; one achieved another CR. All CRS (n=17, 89%) and neurotoxicity (n=4, 21%) events after initial infusion were grades 1-2. The only grade 3 CRS/neurotoxicity and the only high-grade immune effector cell-associated hemophagocytic lymphohistocytosis-like syndrome occurred in the retreatment setting. In vivo cellular kinetic data revealed robust CART22-65s proliferation by quantitative PCR peaking at a median of 20 days postinfusion, with the cells persisting out to month 42 in one patient who achieved a long-term remission with CART22-65s alone.
Conclusions: The favorable safety profile and high remission rates in exceedingly refractory B-ALL support the continued development of CART22-65s but also highlight the need to use the product in combination with HCT or other novel strategies.
Trial registration numbers: NCT02650414 and NCT03620058.
Keywords: Chimeric antigen receptor - CAR; Cytokine release syndrome; Immunotherapy; Leukemia; Relapse.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: CD has consulted for Merck. EOH has consulting or advisory roles with Blueprint Medicines, Cabaletta Bio, and American Board of Internal Medicine Subspecialty Board and has received research funding from DISC medicines, Abbvie Oncology. JLB is employed by, holds stock options with, and holds patents with Novartis BioMedical Research. JJ consults for BlueWhale Bio and is listed on patents managed according to University of Pennsylvania policy. SRR consults for Abbvie and Pfizer. JF holds patents and intellectual property in T-cell-based cancer immunotherapy with royalties; receives research funding from Tmunity Therapeutics and Danaher Corporation; consults for Retro Biosciences; and serves on scientific advisory boards for Cartography Bio, Shennon Biotechnologies, CellFe Biotech, OverT Bio, and Tceleron Therapeutics. MR holds multiple patents related to CART immunotherapy that are managed by the University of Pennsylvania; has consulted for nanoString, BMS, GSK, Bayer, GLG, Guidepoint, Lumicks, and AbClon; receives research funding from AbClon, Beckman Coulter, Lumicks, and Oxford Nano Imaging; and founded viTToria Biotherapeutics. CHJ receives royalties paid from Novartis and Kite to the University of Pennsylvania; is the scientific cofounder and equity holder in Capstan Therapeutics, Dispatch Biotherapeutics and BlueWhale Bio; has board membership with AC Immune; and has scientific advisory roles with various companies, including BlueSphereBio, Cabaletta, Carisma, Cartography, Cellares, Cellcarta, Celldex, Danaher, Decheng, ImmuneSensor, Kite, Poseida, Verismo, Viracta and WIRB-Copernicus group. SLM has received clinical trial support from Novartis and Wugen, has served on advisory boards or study steering committees for Novartis, Wugen, and Syndax, and has a patent pending and licensed to Novartis Pharmaceuticals without royalty for PCT/US2017/044425: Combination Therapies of Car and PD-1 Inhibitors. NVF has consulted for Kite Pharma and Autolus. SAG receives research funding from Cellectis, Kite (a GILEAD company), Novartis, Servier and Vertex; consults for Adaptive Biotech, Eureka Therapeutics, Estrella Immunopharma, Jazz Pharmaceuticals and Novartis; has advised for Allogene, Cabaletta Bio, Jazz Pharmaceuticals, Verismo, Novartis and Vertex and has patents managed according to U Penn and CHOP policies. All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
