Shp-1 regulates the activity of low-affinity T cells specific to endogenous self-antigen during melanoma tumor growth and drives resistance to immune checkpoint inhibition
- PMID: 40246583
- PMCID: PMC12007028
- DOI: 10.1136/jitc-2024-010879
Shp-1 regulates the activity of low-affinity T cells specific to endogenous self-antigen during melanoma tumor growth and drives resistance to immune checkpoint inhibition
Abstract
Background: The presence of activated CD8 T cells in the tumor microenvironment is correlated with an effective immune response to immune checkpoint inhibitor (ICI) therapy. However, ICI predominantly targets high-affinity T cells, which may be less abundant in tumors with few neoantigens. Targeting the intracellular phosphatase Src homology region 2 domain-containing phosphatase-1 (Shp-1) in combination with ICI lowers the T cell activation threshold and enhances the ability of low-affinity T cells to mount a productive antitumor response.
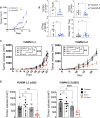
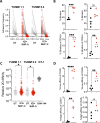
Methods: In this study, we sought to determine whether temporal inhibition of Shp-1 during active tumor growth could rescue the activity of low-affinity T cells specific for endogenous self-antigens. To address this question, we implanted Yale University Mouse Melanoma (YUMM) tumor cell lines into WT mice and, on tumor establishment, administered an inhibitor of Shp-1 (TPI-1) with or without ICI treatment. We analyzed treatment-dependent changes in the immune infiltrate in the tumor via flow cytometry, major histocompatibility complex (MHC) tetramer-mediated detection of tyrosinase-related protein 2 (TRP-2)180-188-specific T cells and a micropipette-based two-dimensional affinity assay to measure the T cell receptor (TCR) affinity.
Results: Administration of ICI and a Shp-1 inhibitor to mice with established YUMM tumors, but neither agent alone, resulted in a significant delay in tumor growth and an increased frequency of CD8 tumor-infiltrating T cells with enhanced effector and reduced exhaustion characteristics. In particular, combined treatment increased the frequency of CD8 T cells specific for the MHC Class I-restricted tumor self-antigen TRP-2180-188. We found that the increase in effector T cells was almost entirely due to an increase in T cells with very low TCR affinity.
Conclusions: We conclude that approaches for altering TCR signaling threshold are effective in enhancing the antitumor response of low-affinity T cells specific for endogenous self-antigens in settings of ICI resistance and/or where neoantigens are not available to drive antitumor responses.
Keywords: Immune Checkpoint Inhibitor; Immunotherapy; Melanoma; T cell; T cell Receptor - TCR.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
