SLC25A42 promotes gastric cancer growth by conferring ferroptosis resistance through enhancing CPT2-mediated fatty acid oxidation
- PMID: 40246810
- PMCID: PMC12006318
- DOI: 10.1038/s41419-025-07644-7
SLC25A42 promotes gastric cancer growth by conferring ferroptosis resistance through enhancing CPT2-mediated fatty acid oxidation
Abstract
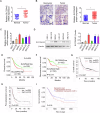
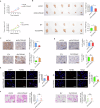
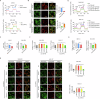
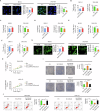
Accumulating evidence has shown that the dysfunction of mitochondria, the multifunctional organelles in various cellular processes, is a pivotal event in the development of various diseases, including human cancers. Solute Carrier Family 25 Member 42 (SLC25A42) is a mitochondrial protein governing the transport of coenzyme A (CoA). However, the biological roles of SLC25A42 in human cancers are still unexplored. Here we uncovered that SLC25A42 is upregulated and correlated with a worse prognosis in GC patients. SLC25A42 promotes the proliferation of gastric cancer (GC) cells while suppresses apoptosis in vitro and in vivo. Mechanistically, SLC25A42 promotes the growth and inhibits apoptosis of GC cells by reprograming lipid metabolism. On the one hand, SLC25A42 enhances fatty acid oxidation-mediated mitochondrial respiration to provide energy for cell survival. On the other hand, SLC25A42 decreases the levels of free fatty acids and ROS to inhibit ferroptosis. Moreover, we found that SLC25A42 reprograms lipid metabolism in GC cells by upregulating the acetylation and thus the expression of CPT2. Collectively, our data reveal a critical oncogenic role of SLC25A42 in GCs and suggest that SLC25A42 represent a promising therapeutic target for GC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study has been approved by the Ethics Committee of Tangdu Hospital. Written informed consents have been collected from each GC patients.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

