RNase1-driven ALK-activation is an oncogenic driver and therapeutic target in non-small cell lung cancer
- PMID: 40246819
- PMCID: PMC12006399
- DOI: 10.1038/s41392-025-02206-x
RNase1-driven ALK-activation is an oncogenic driver and therapeutic target in non-small cell lung cancer
Abstract
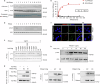
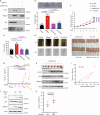
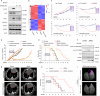
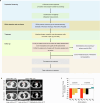
Targeted therapy has achieved significant success in the treatment of non-small cell lung cancer (NSCLC), particularly in patients harboring common oncogenic driver mutations such as EGFR, KRAS, and ALK rearrangement. However, ~35-50% of NSCLC patients without tyrosine kinase mutation or rearrangement (non-mutated) cannot benefit from these targeted treatments, highlighting the urgent need for novel therapeutic strategies for this patient population. In this study, we report a non-canonical role of human secretory ribonuclease 1 (RNase1), which binds to and activates wild-type ALK in lung cancer cells, thereby triggering its downstream signaling pathway. RNase1-driven ALK-activation (RDAA) cells exhibit enhanced cell proliferation, migration, and colony formation. Additionally, RDAA facilitates tumor formation in fibroblast models, further underscoring its oncogenic potential in vivo. Importantly, RDAA lung cancer cells exhibit marked sensitivity to FDA-approved ALK inhibitors. Tumor growth suppression and survival were substantially improved in both RDAA-positive NSCLC cell line-derived and patient-derived xenograft tumor models treated with ALK inhibitors. Monoclonal antibodies against RNase1 and phosphorylated-ALK were used to analyze two different human NSCLC tissue cohorts by immunohistochemical staining identified 10.4% (5/48) and 8.5% (100/1173) patients who were RDAA positive, respectively. Notably, among the nine RDAA-positive NSCLC patients who accepted ALK inhibitor treatment, five achieved objective response including two who experienced complete response (CR). Together, the current study identifies RDAA as an oncogenic driver and proposes an effective targeted therapy strategy for non-mutated NSCLC patients.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer.Cancer Discov. 2013 Apr;3(4):430-43. doi: 10.1158/2159-8290.CD-12-0440. Epub 2013 Mar 26. Cancer Discov. 2013. PMID: 23533265 Free PMC article. Clinical Trial.
-
Genomic Signature of Driver Genes Identified by Target Next-Generation Sequencing in Chinese Non-Small Cell Lung Cancer.Oncologist. 2019 Nov;24(11):e1070-e1081. doi: 10.1634/theoncologist.2018-0572. Epub 2019 Mar 22. Oncologist. 2019. PMID: 30902917 Free PMC article.
-
ALK inhibitors: a new targeted therapy in the treatment of advanced NSCLC.Target Oncol. 2013 Mar;8(1):55-67. doi: 10.1007/s11523-012-0250-9. Epub 2013 Jan 17. Target Oncol. 2013. PMID: 23325296 Review.
-
ALK inhibitors in the treatment of advanced NSCLC.Cancer Treat Rev. 2014 Mar;40(2):300-6. doi: 10.1016/j.ctrv.2013.07.002. Epub 2013 Aug 7. Cancer Treat Rev. 2014. PMID: 23931927 Review.
-
Antitumor activity of alectinib, a selective ALK inhibitor, in an ALK-positive NSCLC cell line harboring G1269A mutation: Efficacy of alectinib against ALK G1269A mutated cells.Cancer Chemother Pharmacol. 2016 Mar;77(3):623-8. doi: 10.1007/s00280-016-2977-y. Epub 2016 Feb 5. Cancer Chemother Pharmacol. 2016. PMID: 26849637
References
-
- Herbst, R. S., Morgensztern, D. & Boshoff, C. The biology and management of non-small cell lung cancer. Nature553, 446–454 (2018). - PubMed
-
- Wu, Y. L. et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med.383, 1711–1723 (2020). - PubMed
-
- Shaw, A. T. et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med.383, 2018–2029 (2020). - PubMed
-
- Duma, N., Santana-Davila, R. & Molina, J. R. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc.94, 1623–1640 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

