The essential calcium channel of sperm CatSper is temperature-gated
- PMID: 40246904
- PMCID: PMC12006431
- DOI: 10.1038/s41467-025-58824-0
The essential calcium channel of sperm CatSper is temperature-gated
Abstract
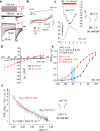
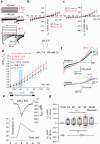
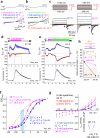
The flagellar calcium channel CatSper is essential for male fertility, as it regulates calcium influx to trigger the hyperactive motility required for sperm to fertilize the egg. Precise activation of CatSper is critical, as premature activation can impair sperm function. While optimal temperature is known to influence fertilization, its effect on CatSper remains unknown. By directly recording from mouse spermatozoa, we reveal that CatSper functions as a temperature-gated ion channel, with a thermal threshold of 33.5 °C and a temperature coefficient Q10 of 5.1. Additionally, we show that physiological levels of spermine reversibly inhibit CatSper's temperature gating, protecting against premature activation. Our findings highlight for the first time the presence of the temperature-gating modality of CatSper and reveal the protective role of spermine, a major component of seminal plasma. These results emphasize the need to maintain testes below 34 °C for optimal fertility and advance understanding of CatSper regulation in male fertility.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors have no competing interests.
Figures




References
-
- Austin, C. R. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B4, 581–596 (1951). - PubMed
-
- Chang, M. C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature168, 697–698 (1951). - PubMed
-
- Suarez, S. S. Control of hyperactivation in sperm. Hum. Reprod. Update14, 647–657 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

