Effect of natural carbonates on microbially induced calcite precipitation process
- PMID: 40246949
- PMCID: PMC12006512
- DOI: 10.1038/s41598-025-97737-2
Effect of natural carbonates on microbially induced calcite precipitation process
Abstract
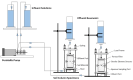
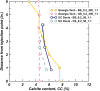
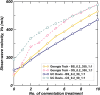
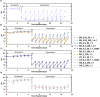
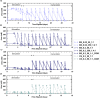
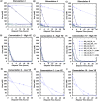
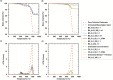
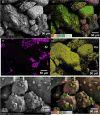
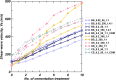
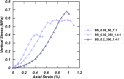
Microbially induced calcite precipitation (MICP) is an emerging ground improvement technique that uses microbes to induce cementation between soil particles. To date, the majority of research has focused on exploring MICP with silica-rich sands; however, the present study investigates the process and efficacy of MICP in a carbonate-rich natural soil, and a comparison is made with benchmark silica-rich sands. MICP column experiments were performed with a range of treatment formulations to optimize and understand the MICP process in carbonate-rich soil. Performance was quantified using chemical (pH, urea, and ammonium concentrations) and physical measurements (TGA and LOI tests). Micro-scale characterization of the cemented soils was performed with XRD, SEM, and EDS, while shear-wave velocity (Vs) and unconfined compressive strength tests were performed to evaluate the effect of precipitated calcite on macroscopic engineering properties. Natural carbonates were found to have a significant impact on the MICP process, resulting in an increase in MICP efficiency of 23% and increases in precipitated calcite contents by as much as 82% when compared to benchmark silica-rich soils receiving similar treatments. These results suggest that the presence of natural carbonate minerals within soils may lower the energy barrier and act as preferential sites for calcite precipitation during the MICP process. Furthermore, SEM images highlighted the association of bacterial cells with precipitated calcite crystals, differences in calcite morphologies and more widespread cementation bonds in carbonate-rich soil when compared to silica sand. Generated cementation also resulted in a linear increase in Vs with increases in precipitated calcite contents for MICP treated carbonate-rich soil, consistent with past results for silica sands. Lastly, differences in yeast extract concentrations applied in treatment solutions were also found to significantly impact the development of ureolytic microbial capacity and the efficiency of the MICP process in the considered soils.
Keywords: Chemical measurements; Microbially induced calcite precipitation; Microscale characterization; Natural carbonates; Shear-wave velocity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- Huang, L., Krigsvoll, G., Johansen, F., Liu, Y. & Zhang, X. Carbon emission of global construction sector. Renew. Sustain. Energy Rev.81, 1906–1916 (2018).
-
- Castro-Alonso, M. J. et al. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: Microbiological and molecular concepts. Front. Mater.10.3389/fmats.2019.00126 (2019).
-
- Mujah, D., Shahin, M. A. & Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J.34, 524–537 (2017).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

