Combining RNA-seq, molecular docking and experimental verification to explore the mechanism of BAM15 as a potential drug for atherosclerosis
- PMID: 40247008
- PMCID: PMC12006321
- DOI: 10.1038/s41598-025-98209-3
Combining RNA-seq, molecular docking and experimental verification to explore the mechanism of BAM15 as a potential drug for atherosclerosis
Abstract
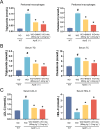
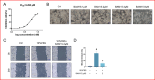
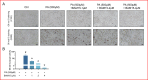
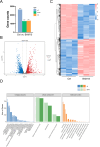
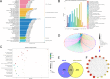
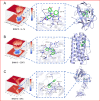
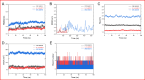
BAM15 is a novel mitochondrial uncoupling agent derived from a synthetic source, that has been wildly explored for its ability to enhance mitochondrial respiration and metabolic flexibility. In this study, we investigated the underlying mechanisms of BAM15 on atherosclerosis (AS) through experimental validation, RNA-seq and molecular docking. The results showed that oral administration of BAM15 suppressed atherosclerosis in western diet (WD)-fed ApoE(-/-) mice and significantly improved the hyperlipidemia. And the increased serum ALT, AST and liver TC, TG, ALT, AST in ApoE(-/-) mice were reduced by BAM15 treatment. In in vitro experiments BAM15 inhibited RAW264.7 macrophages invasive ability and reduced palmitic acid-induced lipid accumulation. RNA-seq results confirmed the differential genes after BAM15 treatment and 140 common targets were identified by intersecting with AS-related targets. A protein-protein interaction (PPI) network analysis high-lighted IL1A, SRC and CSF3 as key targets of BAM15 against AS, which is further verified by molecular docking and western blot. Molecular dynamics analysis results confirmed that BAM15 exhibits strong affinity with the IL-1α, SRC and CSF3 proteins. This study indicates that BAM15 inhibits atherosclerosis through a multi-molecular mechanism, and we propose it as a novel anti-atherosclerotic drug.
Keywords: Atherosclerosis; BAM15; Molecular docking; Molecular mechanism; RNA-seq.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: We confirmed that all experiments were performed in accordance with relevant named guidelines and regulations. And the authors complied with the ARRIVE guidelines for animal experiments. The animal study protocol was approved by the Institutional Review Board of Jiangnan University Medical Center (protocol code 2021-Y-51, 31-Aug-2021). Competing interests: The authors declare no competing interests.
Figures




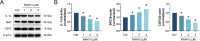
References
MeSH terms
Substances
Grants and funding
- Y20222018/Science and Technology Development Funds of Wuxi
- Y20222021/Science and Technology Development Funds of Wuxi
- Y20212029/Science and Technology Development Funds of Wuxi
- Y20212005/Science and Technology Development Funds of Wuxi
- Y20212031/Science and Technology Development Funds of Wuxi
- 82300510/National Natural Science Foundation of China
- Q202223/Scientific Research Program of Wuxi Health Commission
- Q202227/Scientific Research Program of Wuxi Health Commission
- Q202329/Scientific Research Program of Wuxi Health Commission
- CXTDPY2021001/Key Subject Medical Innovation Team of Wuxi
- BK20210066/Natural Science Foundation of Jiangsu Province
- HB2023038/Top Talent Support Program for young and middle-aged people of Wuxi Health Committee
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

