Non-invasive screening for liver fibrosis by acoustic radiation force impulse in patients with ciliopathies
- PMID: 40247009
- PMCID: PMC12006320
- DOI: 10.1038/s41598-025-96246-6
Non-invasive screening for liver fibrosis by acoustic radiation force impulse in patients with ciliopathies
Abstract
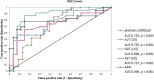
Primary cilia are antenna-like structures on the surface of epithelial cells involved in multiple signaling pathways. Their malfunction can cause a heterogenous group of diseases called ciliopathies with a broad spectrum of organ involvements, including liver fibrosis. The aim of this exploratory study was to evaluate elastography measurement via ultrasound based acoustic radiation force impulse imaging (ARFI) as a screening tool for liver fibrosis in ciliopathies. In a prospective cohort of 51 patients with ciliopathies (aged between 2 months and 66 years) from the NEOCYST registry stiffness of the right liver lobe and spleen was measured via ARFI and results were then compared with laboratory parameters, endoscopic, ultrasonographic and histological findings. ARFI screening of the liver identified 27 patients without increased liver stiffness suggesting no or insignificant fibrosis, 11 with intermediate fibrosis, and 12 with liver fibrosis F4. Four patients showed increased spleen stiffness in ARFI. In all 10 patients with histologically confirmed fibrosis, ARFI results perfectly matched fibrosis stages. In the ARFI-based overall fibrosis subgroup, ALT, AST, GGT and spleen size were significantly increased, whereas platelets were significantly decreased compared to the no fibrosis subgroup. Normal GGT excluded ARFI-defined F4 fibrosis (negative predictive value 100%). Gene variants in PKHD1, TMEM67, and TULP3 were primarily detected in our patients with liver fibrosis whereas NPHP1 and HNF1B were not associated with increased liver stiffness. ARFI is a valuable screening tool for the detection of liver involvement in ciliopathies and may be useful in addition to laboratory and clinical parameters alone.Trial registration: NEOCYST registry DRKS00011003, registered 06.09.2016, https://drks.de/search/en/trial/DRKS00011003 .
Keywords: Ciliopathies; Congenital hepatic fibrosis; Liver elastography; Liver stiffness measurement; Spleen stiffness measurement.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The trial was approved by Muenster University hospitals’ ethics committee (AZ2016-284-f-S) and was conducted in compliance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines and local regulatory requirements. All participating patients or legal guardians (for children) gave written consent for the publication of their anonymized data.
Figures


References
-
- Davenport, J. R. & Yoder, B. K. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am. J. Physiology-Renal Physiol.289 (6), F1159–F1169. 10.1152/ajprenal.00118.2005 (2005). - PubMed
-
- McConnachie, D. J., Stow, J. L. & Mallett, A. J. Ciliopathies and the kidney: A review. Am. J. Kidney Dis. Official J. Natl. Kidney Foundation. 77 (3), 410–419. 10.1053/j.ajkd.2020.08.012 (2021). - PubMed
-
- Desmet, V. J. Congenital diseases of intrahepatic bile ducts: variations on the theme ductal plate malformation. Hepatology16 (4), 1069–1083. 10.1002/hep.1840160434 (1992). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

