Circulating innate lymphoid cells are dysregulated in patients with prostate cancer
- PMID: 40247153
- PMCID: PMC12007220
- DOI: 10.1186/s11658-025-00725-7
Circulating innate lymphoid cells are dysregulated in patients with prostate cancer
Abstract
Background: Prostate cancer (PCa) is the second most common cancer affecting men globally, especially those aged 50 years and above. Despite substantial progress in terms of both prognosis and therapy, PCa remains a significant health concern, necessitating the identification of novel therapeutic targets. Innate lymphoid cells (ILCs) have emerged as critical modulators of tumor immunity, exhibiting both pro- and antitumoral effects. However, little is known yet about their contribution in PCa. This study investigated the phenotypic and functional profiles of ILC subsets in the peripheral blood mononuclear cells (PBMCs) of patients with PCa stratified by Gleason score.
Methods: PBMCs were isolated by Lymphoprep. ILC frequency and activity were evaluated by flow cytometry. The levels of ILC-activating cytokines were analyzed by multiplex assay in the serum of healthy donors (HDs) and patients with PCa. To evaluate the crosstalk between ILC2s and cancer cells, PC3 and DU145 human PCa cell lines were used.
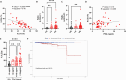
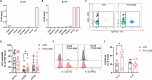
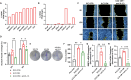
Results: We found a stage-dependent increase in the protumoral ILC2 frequency and a concurrent decrease in antitumoral ILC1s in patients with PCa compared with healthy controls. Interestingly, the frequency of ILC2s was higher in patients with elevated prostate-specific antigen (PSA) values, suggesting their potential as molecular predictor for defining the risk category of patients with PCa at diagnosis. Importantly, patients with PCa exhibited hyperactivated ILC2s, characterized by elevated interleukin (IL)-13 and IL-5 production, while ILC1s displayed reduced tumor necrosis factor (TNF)-α and interferon (IFN)-γ secretion. Furthermore, serum levels of ILC2-activating cytokines IL-33, IL-18, and prostaglandin D2 (PGD2) were elevated in patients with PCa. In vitro co-culture experiments demonstrated that PCa cell lines, capable of secreting these cytokines, could directly enhance ILC2 activity. Likewise, ILC2-derived IL-13 promoted PCa cell migration and invasion.
Conclusions: Collectively, our findings highlight a dysregulated ILC profile in PCa, characterized by ILC2 dominance and heightened activity at the expense of ILC1s, suggesting both ILC1s and ILC2s as potential therapeutic targets for PCa treatment.
Keywords: IL-13; IL-18; IL-33; ILC1s; ILC2s; Innate lymphoid cells; Prostate cancer.
© 2025. The Author(s).
Conflict of interest statement
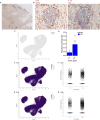
Declarations. Ethics approval and consent to participate: Venous blood was drawn from HDs and patients with PCa at the University Hospital Federico II in Naples, between September 2021 and December 2023. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). The study protocol was approved by the Ethics Committee of the University Hospital Federico II in Naples (approval no. 180/20). All participants provided informed consent before participating in the study. For IHC, human prostate tissues were provided by the Tissue Bank Bern. Analyses of the samples were approved by the Cantonal Ethics Committee of Bern (200/2014 and 2024-02031). Consent for publication: Not applicable. Competing interests: The authors declare no potential competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–16. - PubMed
-
- Catalona WJ, Hudson MA, Scardino PT, Richie JP, Ahmann FR, Flanigan RC, et al. Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152:2037–42. - PubMed
-
- Dalton DL. Elevated serum prostate-specific antigen due to acute bacterial prostatitis. Urology. 1989;33:465. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

