Diprovocim protects against the radiation-induced damage via the TLR2 signaling pathway
- PMID: 40247162
- PMCID: PMC12004591
- DOI: 10.1186/s10020-025-01198-2
Diprovocim protects against the radiation-induced damage via the TLR2 signaling pathway
Abstract
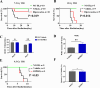
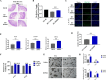
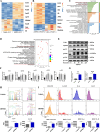
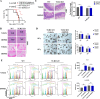
Severe ionizing radiation (IR) causes the acute lethal damage of hematopoietic system and gastrointestinal tract. By establishing a radiation injury model, we found that Diprovocim, a TLR2 agonist, protected mice against the lethal damage of hematopoietic system and gastrointestinal tract. Diprovocim inhibited the IR-induced damage, promoted erythrocyte differentiation and elevated the proportion of hematopoietic stem cells (HSCs) in irradiated mice, and promoted the proliferation and differentiation of intestinal stem cells (ISCs). In addition, the RNA seq results suggested that Diprovocim significantly upregulated the TLR2 signaling pathway, and Diprovocim had no radioprotective effect on TLR2 KO mice, suggesting that Diprovocim activated TLR2 signaling pathway to exert its radioprotective function. The RNA sequencing results also suggested that Diprovocim significantly up-regulated the expression of SOX9. Diprovocim had no radioprotective effect after SOX9 knockdown. In conclusion, we demonstrated that Diprovocim protected the radiation-induced damage and upregulated targeting TLR2-SOX9 axis and that Diprovocim might be a potential high-efficiency selective agent.
Keywords: Diprovocim; Intestinal stem cells; Ionizing radiation-induced injury; SOX9; TLR2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals'(NIH Publication No. 85–23, National Academy Press, Washington, DC, revised 1996), with the approval of the Laboratory Animal Center of the Naval Medical University, Shanghai. Consent for publication: Written informed consent for publication was obtained from all participants. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Zymosan-a Protects the Hematopoietic System from Radiation-Induced Damage by Targeting TLR2 Signaling Pathway.Cell Physiol Biochem. 2017;43(2):457-464. doi: 10.1159/000480472. Epub 2017 Sep 1. Cell Physiol Biochem. 2017. PMID: 28922655
-
Novel chimeric TLR2/NOD2 agonist CL429 exhibited significant radioprotective effects in mice.J Cell Mol Med. 2021 Apr;25(8):3785-3792. doi: 10.1111/jcmm.16252. Epub 2021 Feb 19. J Cell Mol Med. 2021. PMID: 33609010 Free PMC article.
-
Structural Basis of TLR2/TLR1 Activation by the Synthetic Agonist Diprovocim.J Med Chem. 2019 Mar 28;62(6):2938-2949. doi: 10.1021/acs.jmedchem.8b01583. Epub 2019 Mar 13. J Med Chem. 2019. PMID: 30829478 Free PMC article.
-
Radioprotective agents against the ionizing radiation-induced hematopoietic stem and progenitor cell injury; Foundation review.Crit Rev Oncol Hematol. 2025 Jul;211:104713. doi: 10.1016/j.critrevonc.2025.104713. Epub 2025 Apr 3. Crit Rev Oncol Hematol. 2025. PMID: 40187710 Review.
-
Hematopoietic stem cell injury induced by ionizing radiation.Antioxid Redox Signal. 2014 Mar 20;20(9):1447-62. doi: 10.1089/ars.2013.5635. Epub 2014 Feb 10. Antioxid Redox Signal. 2014. PMID: 24124731 Free PMC article. Review.
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. 10.1016/j.cell.2006.02.015. - PubMed
-
- Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. 10.1371/journal.ppat.1000902. - PMC - PubMed
-
- Brickey WJ, Caudell DL, Macintyre AN, Olson JD, Dai Y, Li S, Dugan GO, Bourland JD, O’Donnell LM, Tooze JA, et al. The TLR2/TLR6 ligand FSL-1 mitigates radiation-induced hematopoietic injury in mice and nonhuman primates. Proc Natl Acad Sci U S A. 2023;120:e2122178120. 10.1073/pnas.2122178120. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

