Assessment of functional recovery in patients with schizophrenia, with a focus on early-phase disease: results from a Delphi consensus and narrative review
- PMID: 40247199
- PMCID: PMC12007312
- DOI: 10.1186/s12888-025-06725-3
Assessment of functional recovery in patients with schizophrenia, with a focus on early-phase disease: results from a Delphi consensus and narrative review
Abstract
Background: Treatment of schizophrenia has traditionally aimed for symptomatic remission without addressing many daily problems patients face. Although no standard definition of functional recovery in patients with first-episode psychosis (FEP) and early-phase (EP) schizophrenia exists in the literature, most clinicians consider it a useful concept in daily practice. We conducted a Delphi panel to develop expert consensus on assessing functional recovery in FEP and EP schizophrenia patients and defining its domains, which we compared with currently available patient- and clinician-reported outcome measures (PROMs, CROMs).
Methods: The three-stage modified Delphi panel consisted of a 1:1 interview round and two online survey rounds involving five expert steering committee and 16 panel members. We conducted a narrative review of the literature in PubMed to identify instruments assessing functioning in people with schizophrenia.
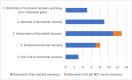
Results: Panelists were presented with 38 statements about functional recovery (definition, domains, and assessment) and approaches to achieving it. Panelists defined functional recovery for FEP and EP schizophrenia patients as a multidimensional state closely related to quality of life. When evaluating functional recovery, panelists agreed that assessing (1) depression, (2) aggressive behavior, (3) social interaction, (4) family functioning, (5) education and/or employment, (6) leisure activities, (7) self-care, and (8) sexual functioning was important. Panelists also agreed that asking patients about self-care and sexual functioning was less critical at every encounter. It was agreed that patients may be said to have reached partial functional recovery if they recovered in some but not all domains. There was consensus that long-acting injectable antipsychotics can facilitate functional recovery by increasing treatment adherence, lessening disease and treatment burden, and reducing functional decline. The literature review identified eight PROMs and CROMs that assess functioning in schizophrenia. However, none evaluated all eight domains of functional recovery.
Conclusions: Functional recovery is an important treatment goal in FEP and EP patients. PROMs and CROMs do not evaluate all eight domains of functional recovery agreed by the Delphi panel. Further research is needed to better understand and improve how functional recovery is assessed in clinical practice.
Keywords: Antipsychotics; Clinical consensus; Delphi panel; Early-phase schizophrenia; First-episode psychosis; Functional recovery; Long-acting injectable; Patient-reported outcome measures; Recommendations; Schizophrenia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval for the Delphi panel study was sought from Pearl Pathways (reference: #23-OHTH-106, 30/1/2023), a central Institutional Review Board that reviewed and approved all study material including the protocol. Given the fact that no individual patient data was collected in the course of this study and physician participation was voluntary, this study was determined to be Exempt according to the FDA 21 CFR 56.104 and 45CFR46.104(d)(2). The study was conducted in accordance with the relevant guidelines and regulations for research involving human subjects. Information on study purpose, format, and outputs were provided to the panelists through a market research agreement document. Informed consent was obtained from all panelists before their participation in this Delphi panel. The narrative review was based on previously conducted studies, and ethical approval was therefore deemed not necessary. This study was conducted in accordance with the principles of the Declaration of Helsinki, ensuring ethical standards in research involving human participants. Consent for publication: Not applicable. Competing interests: P.G. received fees during the last 5 years for presentations at congresses or participation in scientific boards from Angelini, EISI, Janssen, Lundbeck, Otsuka, Richter, Merck, and Viatris. M.Y. is a full-time employee of H. Lundbeck A/S. J. M. is a full-time employee of Otsuka Pharmaceutical Development and Commercialization Inc. A. F. is/has been a consultant and/or a speaker and/or has received research grants from Angelini, Apsen, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Italfarmaco, Lundbeck, Janssen, Mylan, Otsuka, Pfizer, Recordati, Sanofi Aventis, Sunovion, Viatris, and Vifor. C.A. has received support by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (ISCIII), co-financed by the European Union, ERDF Funds from the European Commission, “A way of making Europe,” financed by the European Union – NextGenerationEU (PMP21/00051), PI19/01024. CIBERSAM, Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), European Union Structural Funds, European Union Seventh Framework Program, European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking: Project PRISM-2 (Grant agreement No.101034377), Project AIMS-2-TRIALS (Grant agreement No 777394), Horizon Europe, the National Institute of Mental Health of the National Institutes of Health under Award Number 1U01MH124639-01 (Project ProNET) and Award Number 5P50MH115846-03 (project FEP-CAUSAL), Fundación Familia Alonso, and Fundación Alicia Koplowitz. C.A. additionally has been a consultant to or has received honoraria or grants from Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering-Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda. C.U.C. has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Adcock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol Myers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Maplight, Mylan, Neumora Therapeutics, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Saladax, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Tolmar, Vertex, Viatris and Xenon Pharmaceuticals. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Mindpax, and Quantic. J.M.K. has been a consultant for or received honoraria from Alkermes, Allergan, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HealthRhythms, HLS, Indivior, Intracellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Newron, Novartis, Otsuka, Roche, Saladax, Sunovion, and Teva. Dr. Kane has received grant support from Otsuka, Lundbeck, Sunovion and Janssen. Dr. Kane is a shareholder in Vanguard Research Group, North Shore Therapeutics, Health Rhythms, MedinCell, and LB Pharmaceuticals, Inc. N.S. is a former employee of OPEN Health, which received funding from Lundbeck Otsuka Alliance to conduct this study. E.A., A.B., and S.S. are employees of OPEN Health, which received funding from Lundbeck Otsuka Alliance to conduct this study.
Figures


References
-
- Hoseinipalangi Z, Golmohammadi Z, Rafiei S, Pashazadeh Kan F, Hosseinifard H, Rezaei S, Ahmadi S, Ahmadi N, Raoofi S, Aghajani F, et al. Global health-related quality of life in schizophrenia: systematic review and meta-analysis. BMJ Support Palliat Care. 2022;12(2):123–31. - PubMed
-
- Silva MA, Restrepo D. Functional recovery in schizophrenia. Rev Colomb Psiquiatr (Engl Ed). 2019;48(4):252–60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

