Study on effect of pancreatic kininogenase on diabetic nephropathy-induced fibrosis via Notch1/Hes1/Pten/Akt signaling pathway
- PMID: 40247227
- PMCID: PMC12007155
- DOI: 10.1186/s12882-025-04050-1
Study on effect of pancreatic kininogenase on diabetic nephropathy-induced fibrosis via Notch1/Hes1/Pten/Akt signaling pathway
Abstract
Objective: To elucidate the mechanism by which pancreatic kininogenase (PKase) impacts renal fibrosis in diabetic nephropathy through modulation of the Notch1/Hes1 and Pten/Akt pathways.
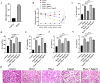
Methods: This study employed in vivo models and cellular assays to investigate PKase's effects on cellular viability, apoptosis, and oxidative stress. Assay kits were used to assess these parameters, while protein expression levels were measured via Western Blot and RT-qPCR. Histological changes in kidney tissues were analyzed using HE and Masson's staining. Fibrosis markers-including E-cadherin, vimentin, α-SMA, Collagen I, TGF-β, and fibronectin-were evaluated through immunofluorescence and immunohistochemistry.
Results: After eight weeks of PKase treatment, significant improvements in blood glucose levels and associated symptoms were observed in diabetic nephropathy rats. Both in vivo and in vitro results demonstrated that PKase treatment inhibited the expression of diabetic nephropathy markers, including vimentin, α-SMA, FN, Collagen I, and TGF-β, while increasing the expression of E-cadherin. Additionally, the expression of Notch1, Hes1, and phosphorylated Akt (p-Akt) was upregulated, and Pten expression was suppressed, all of which were reversed by PKase treatment. Furthermore, both analyses indicated that PKase alleviated Jagged1-induced apoptosis and oxidative stress, and mitigated tubulointerstitial fibrosis.
Conclusion: PKase appears to ameliorate diabetic nephropathy-induced renal fibrosis by activating the Pten/Akt pathway and inhibiting the Notch1/Hes1 pathway, suggesting its potential as a therapeutic agent in diabetic nephropathy.
Clinical trial number: Not applicable.
Keywords: Diabetic nephropathy; Notch1/Hes1 pathway; PKase; Pten/Akt pathway; Renal fibrosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was approved by the ethics committee of The Second Affiliated Hospital, Hengyang Medical School, University of South China (YK2023011). Consent to participate and consent to publish: This study was conducted in accordance with the guidelines provided by the ethics committee and adhered to all relevant ethical standards for the care and use of laboratory animals. The animals used in this study were provided by Huafukang Bio, and informed consent was obtained from all participants prior to their inclusion in the study. Throughout the study, animal care and handling were in compliance with ethical guidelines to ensure their well-being. Competing interests: The authors declare no competing interests.
Figures






References
-
- Xu Y, et al. Diabetic nephropathy execrates epithelial-to-mesenchymal transition (EMT) via miR-2467-3p/Twist1 pathway. Biomed Pharmacother. 2020;125:109920. - PubMed
-
- Wang J, et al. Low molecular weight fucoidan alleviates diabetic nephropathy by binding fibronectin and inhibiting ECM-receptor interaction in human renal mesangial cells. Int J Biol Macromol. 2020;150:304–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

