A roadmap of priority evidence gaps for the co-implementation of malaria vaccines and perennial malaria chemoprevention
- PMID: 40247263
- PMCID: PMC12007247
- DOI: 10.1186/s12936-025-05347-0
A roadmap of priority evidence gaps for the co-implementation of malaria vaccines and perennial malaria chemoprevention
Erratum in
-
Correction: A roadmap of priority evidence gaps for the co-implementation of malaria vaccines and perennial malaria chemoprevention.Malar J. 2025 Jun 24;24(1):199. doi: 10.1186/s12936-025-05410-w. Malar J. 2025. PMID: 40556018 Free PMC article. No abstract available.
Abstract
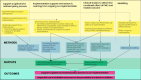
Progress in malaria control will rely on deployment and effective targeting of combinations of interventions, including malaria vaccines and perennial malaria chemoprevention (PMC). Several countries with PMC programmes have introduced malaria vaccination into their essential programmes on immunizations, but empirical evidence on the impact of combining these two interventions and how best to co-implement them are lacking. At the American Society of Tropical Medicine and Hygiene 2023 annual meeting, a stakeholder meeting was convened to identify key policy, operational and research gaps for co-implementation of malaria vaccines and PMC. Participants from 11 endemic countries, including representatives from national malaria and immunization programmes, the World Health Organization, researchers, implementing organizations and funders attended. Identified evidence gaps were prioritized to select urgent issues to inform co-implementation. The output of these activities is a strategic roadmap of priority malaria vaccine and PMC co-implementation evidence gaps, and solutions to address them. The roadmap was presented to stakeholders for feedback at the 2024 Multilateral Initiative on Malaria meeting and revised accordingly. The roadmap outlines four key areas of work to address urgent evidence gaps for co-implementation: (1) support to the global and national policy process, (2) implementation support and research, (3) clinical studies, and (4) modelling. Together, these areas will provide practical guidance on the co-implementation of the interventions, and robust evidence to inform decision-making on how best to design, optimize and scale-up co-implementation in different contexts, including if and in what contexts the co-implementation is cost-effective, and the optimal schedule for co-implementation. This will work towards supporting the policy process on co-implementation of malaria vaccines and PMC, and achieving the most impactful use of available resources for the prevention of malaria in children.
Keywords: Co-implementation; Malaria vaccine; Perennial Malaria Chemoprevention (PMC); R21; RTS,S.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- WHO. World malaria report 2024. Geneva: World Health Organization; 2024.
-
- World Health Organization. Malaria vaccine: WHO position paper—March 2022. Wkly Epidemiol Rec. 2022;97:61–80.
-
- WHO. WHO guidelines for malaria. Geneva: World Health Organization; 2023.
-
- Dicko A, Ouedraogo JB, Zongo I, Sagara I, Cairns M, Yerbanga RS, et al. Seasonal vaccination with RTS,S/AS01E vaccine with or without seasonal malaria chemoprevention in children up to the age of 5 years in Burkina Faso and Mali: a double-blind, randomised, controlled, phase 3 trial. Lancet Infect Dis. 2023;24:75–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

