Nocturnal baricitinib administration leads to rapid drug responses in rheumatoid arthritis: a multicenter non-randomized controlled study
- PMID: 40247352
- PMCID: PMC12004773
- DOI: 10.1186/s13075-025-03555-2
Nocturnal baricitinib administration leads to rapid drug responses in rheumatoid arthritis: a multicenter non-randomized controlled study
Abstract
Background: Inflammatory cytokine levels exhibit a circadian rhythm in sera, peaking from late night to early morning in patients with rheumatoid arthritis (RA). This cytokine kinetics is a recognized therapeutic target. This clinical study aimed to evaluate the effectiveness of night-time baricitinib administration based on cytokine secretion.
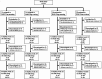
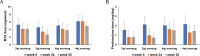
Methods: In this 52-week multicenter non-randomized controlled study, 122 patients with RA were assigned to four groups: baricitinib 2 mg morning (BAR2MORN), 2 mg evening (BAR2EVE), 4 mg morning (BAR4MORN), or 4 mg evening (BAR4EVE). The primary endpoint was assessed using the 20% improvement in the American College of Rheumatology criteria (ACR20) at week 12. The secondary endpoints were ACR20/50/70 and changes in the clinical disease activity index (CDAI) through 52 weeks. The results were evaluated using the propensity score inverse probability of treatment weighted to reduce selection bias in patient background.
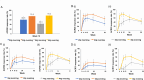
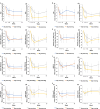
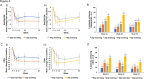
Results: BAR4EVE resulted in better primary endpoint improvement than BAR4MORN (78.2 vs. 43.3%; p < 0.001). No difference in improvement was observed in the primary endpoint between BAR2EVE and BAR2MORN (75.5 vs. 60.6%; p = 0.10). However, BAR2EVE demonstrated higher ACR20 at weeks 4, 24, and 52 and ACR50 at weeks 4 and 12 than BAR2MORN. BAR4EVE demonstrated higher ACR20/50 at weeks 4, 8, and 12 and ACR70 at weeks 8, 12, and 24 than BAR4MORN. CDAI changes were significantly reduced in BAR4EVE than in BAR4MORN at weeks 4 and 8.
Conclusion: Chronotherapy targeting cytokine secretion resulted in rapid drug response, proposing a new potential application for JAK inhibitors.
Trial registration: UMIN000040094, July 1, 2020.
Keywords: Baricitinib; Chronotherapy; JAK inhibitor; Rheumatoid arthritis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Ethics Committees of Hyogo Medical University, Kobe City Medical Center West Hospital, Konan (Hakuhoukai), Kakogawa Hospital, and Kobe Kaisei Hospital. All participants signed informed consent forms. Consent for publication: Not applicable. Competing interests: AH: Honoraria from AbbVie G.K., and honoraria and grants from Eli Lilly Japan K.K., Asahikasei Pharma Co. and Chugai Pharmaceutical Co. TY (Takashi Yamane): Honoraria from GlaxoSmithKline K.K. KM: Grants from Asahikasei Pharma Co. and Chugai Pharmaceutical Co. The other authors: No conflicts of interest.
Figures
References
-
- Olsen NJ, Brooks RH, Furst D. Variability of Immunologic and clinical features in patients with rheumatoid arthritis studied over 24 hours. J Rheumatol. 1993;20:940–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

