TREM2 and sTREM2 in Alzheimer's disease: from mechanisms to therapies
- PMID: 40247363
- PMCID: PMC12004684
- DOI: 10.1186/s13024-025-00834-z
TREM2 and sTREM2 in Alzheimer's disease: from mechanisms to therapies
Abstract
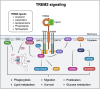
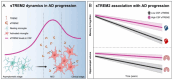
Triggering receptor expressed on myeloid cells 2 (TREM2) is an innate immune receptor predominantly expressed by microglia in the brain. Recent studies have established TREM2 as a central immune signaling hub in neurodegeneration, where it triggers immune responses upon sensing pathological development and tissue damages. TREM2 binds diverse ligands and activates downstream pathways that regulate microglial phagocytosis, inflammatory responses, and metabolic reprogramming. Interestingly, TREM2 exists both in its membrane-bound form and as a soluble variant (sTREM2), that latter is generated through proteolytic shedding or alternative splicing and can be detected in cerebrospinal fluid and plasma. Emerging clinical and preclinical evidence underscores the potential of TREM2 and sTREM2 as diagnostic biomarkers and therapeutic targets in Alzheimer's disease (AD). This review provides a comprehensive overview of the molecular functions, regulatory mechanisms, and pathological implications of TREM2 and sTREM2 in AD. Furthermore, we explore their potential roles in diagnostics and therapeutics while suggesting key research directions for advancing TREM2/sTREM2-based strategies in combating AD.
Keywords: Alzheimer’s disease; Amyloid; Metabolism; Microglia; Neurodegeneration; TREM2; Tau; sTREM2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All the authors are consent to the publication of this study. Competing interests: G.B. consults for SciNeuro Pharmaceuticals and Kisbee Therapeutics. Other authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 32170952/National Natural Science Foundation of China
- 32200805/National Natural Science Foundation of China
- 2024J01613120/Natural Science Foundation of Fujian Province
- JCYJ20240813145512017/Shenzhen Science and Technology Innovation Program
- JCYJ20220530154407016/Shenzhen Science and Technology Innovation Program
LinkOut - more resources
Full Text Sources
Medical

