[Effect of low-dose primaquine treatment on Plasmodium vivax recurrence and transmission-blocking activity in southwest Ethiopia: a longitudinal cohort study
- PMID: 40247375
- PMCID: PMC12004725
- DOI: 10.1186/s12936-025-05365-y
[Effect of low-dose primaquine treatment on Plasmodium vivax recurrence and transmission-blocking activity in southwest Ethiopia: a longitudinal cohort study
Abstract
Background: Existing malaria control strategies for Plasmodium vivax are challenging due to its dormant and relapsing liver stages, as well as the early onset of gametocytogenesis. Primaquine (PQ) effectively eliminates dormant stages and can kill gametocytes; however, it necessitates repeated dosing. In this study, the effectiveness of chloroquine (CQ) plus low-dose of PQ on recurrence and its transmission-blocking activity was evaluated.
Methods: Between September 2019 and July 2022, a prospective cohort study was conducted in the Jimma-Arjo and Dabo-Hanna districts of the Oromia region in Ethiopia. A total of 214 uncomplicated cases of P. vivax malaria were enrolled in the study. Participants were treated with either CQ alone (106) or CQ + PQ (108), based on whether their district was targeted for P. vivax elimination by the national malaria programme or not. After enrolment, participants were followed for clinical illness and parasitaemia on days 28, 42, and monthly for one year. To assess the effect of different treatment regimens on transmission-blocking activity, Anopheles arabiensis mosquitoes were used in direct membrane-feeding assays (DMFA) at baseline (pre-treatment) and on day 42 (post-treatment). Based on polymerase chain reaction (PCR) positivity, the time to the first recurrence was estimated using Kaplan-Meier survival analysis. Cox regression models were employed to assess risk factors for recurrence.
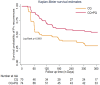
Results: Of 3,590 individuals screened for malaria, 323 tested positive for P. vivax, and 214 were enrolled. Of these, 98.6% (211/214) completed the day 28 follow-up, and 67.3% (144/214) completed the one-year follow-up. Between days 28 and 42, 24% (95% CI 15.8-32.2%) of those individuals receiving CQ alone were PCR positive, and 10.3% (95% CI 4.5-16.0%) in those receiving CQ plus PQ. This represented a 57.3% reduction P. vivax recurrence in the CQ + PQ treatment group compared to CQ alone (risk ratio = 0.427, 95% CI 0.222-0.824, p = 0.008). During the year of follow-up at least one recurrence occurred in 70% (95% CI 59.1-80.2%) of the CQ alone and 46% (95% CI 35.5-58.1%) in the CQ + PQ treatment group (p < 0.001). Treatment regimen, high baseline parasitaemia and presence of gametocytaemia were risk factors for P. vivax recurrence. Compared to baseline DMFA at day 42, individuals showed an inhibition intensity of 39.0% in the CQ alone versus 77.8% in the CQ + PQ treatment group (p = 0.016), while inhibition prevalence was 35.2% in the CQ alone and 70.1% in the CQ + PQ treatment group (p = 0.021).
Conclusions: This study demonstrate that with limited supervision of CQ + PQ treatment significantly lowers the risk of P. vivax recurrence in health clinics of southwest Ethiopia compared to CQ alone. Adding PQ to CQ also reduced P. vivax transmission to mosquito vectors relative to CQ alone but did not result in a complete transmission-blocking effect by day 42 post-treatment. Therefore, improved health education on treatment adherence and bed net use could enhance the effectiveness of PQ plus CQ. Higher doses of PQ for a shorter duration may be necessary to enhance treatment adherence, reduce recurrence rates, and decrease the risk of transmission.
Keywords: Plasmodium vivax; Chloroquine; Primaquine; Recurrent; Transmission-blocking activity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
In vivo efficacy of chloroquine plus primaquine combination therapy against uncomplicated Plasmodium vivax malaria in Limu Kossa District, Jimma Zone, Southwest Ethiopia.Malar J. 2024 Oct 8;23(1):300. doi: 10.1186/s12936-024-05124-5. Malar J. 2024. PMID: 39380029 Free PMC article. Clinical Trial.
-
Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax infection in Ethiopia: A randomized controlled trial.PLoS Med. 2017 May 16;14(5):e1002299. doi: 10.1371/journal.pmed.1002299. eCollection 2017 May. PLoS Med. 2017. PMID: 28510573 Free PMC article. Clinical Trial.
-
Efficacy of chloroquine with primaquine for uncomplicated Plasmodium vivax malaria without G6PD testing in Northwest Ethiopia: a one-arm in vivo prospective therapeutic efficacy study.Malar J. 2025 Jun 15;24(1):192. doi: 10.1186/s12936-025-05446-y. Malar J. 2025. PMID: 40518540 Free PMC article.
-
In vivo efficacy of anti-malarial drugs against clinical Plasmodium vivax malaria in Ethiopia: a systematic review and meta-analysis.Malar J. 2021 Dec 24;20(1):483. doi: 10.1186/s12936-021-04016-2. Malar J. 2021. PMID: 34952581 Free PMC article.
-
Tafenoquine for preventing relapse in people with Plasmodium vivax malaria.Cochrane Database Syst Rev. 2020 Sep 6;9(9):CD010458. doi: 10.1002/14651858.CD010458.pub3. Cochrane Database Syst Rev. 2020. PMID: 32892362 Free PMC article.
Cited by
-
Efficacy of Focal Primaquine Mass Administration for Eliminating Plasmodium vivax Malaria in Northern Myanmar: A Cluster-Randomized Trial.Open Forum Infect Dis. 2025 Aug 5;12(8):ofaf465. doi: 10.1093/ofid/ofaf465. eCollection 2025 Aug. Open Forum Infect Dis. 2025. PMID: 40799786 Free PMC article.
References
-
- WHO. World malaria report 2023. Geneva: World Health Organization; 2023.
-
- Federal Democratic Republic of Ethiopia Ministry of Health. National Malaria Guidelines, 4th Edn. Addis Ababa; 2017.
-
- Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

