KRAS mutation detection by liquid biopsy for pancreatic ductal adenocarcinoma
- PMID: 40247392
- PMCID: PMC12004821
- DOI: 10.1186/s13045-025-01696-0
KRAS mutation detection by liquid biopsy for pancreatic ductal adenocarcinoma
Abstract
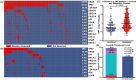
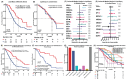
The clinical utility of liquid biopsy (LB) for pancreatic ductal adenocarcinoma (PDAC) remain understudied. Our single-institution cohort of 311 PDAC patients with non-tumor tissues informed LB found 81.2% positivity (N = 186) in metastatic cases and in 52.4% (N = 43) of localized disease. KRAS mutations were detected in 64.6% (N = 148) of metastatic cases and 16% (N = 13) for localized disease. Positive LB, especially KRAS mutation detection, is associated with worse overall survival (OS) in metastatic PDAC (median 14.5 vs. 31.3 months, HR = 2.7, 95%CI = 1.7-4.3, P < 0.0001). The positive concordance rates of KRAS and TP53 mutations were 63% and 68% in metastatic disease but only 7% (KRAS) and 33% (TP53) in localized disease, respectively. Among the 41 patients who underwent serial liquid biopsy testing, 25% tested positive after an initial negative result. LB detects therapeutically targetable mutations in 58.5% of PDAC patients and is associated with OS.
Keywords: KRAS; Liquid biopsy; Molecular profiling; Mutation; OS; PDAC; Pancreatic cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the MD Anderson Institutional Review Board (IRB), protocol number 2023-0091. A waiver of informed consent was granted per the USA federal regulation 45 CFR 46.116(f) for this retrospective study. Competing interests: Brandon Smaglo: Consulting for Ipsen.Shubham Pant: Advisory for Zymeworks, Ipsen, Novartis, Janssen, Boehringer Ingelheim, and AskGene Pharma; and he receives research funding from Mirati Therapeutics, Lilly, Xencor, Novartis, Rgenix, Bristol-Myers Squibb, Astellas Pharma, Framewave, 4D Phar-ma, Boehringer Ingelheim, NGM Biopharmaceuticals, Janssen, Arcus Biosciences, Elicio Therapeutics, Bionte, Ipsen, Zymeworks, Pfizer, ImmunoMET, Imuneering, and Amal Therapeutics. Anirban Maitra: Consultant for Tezcat Biosciences is listed as an inventor of a patent licensed to Thrive Earlier Detection (an Exact Sciences Company) relevant to early detection of pancreatic cancer. John Paul Shen: Grant/research support/collaboration: Celsius Therapeutics, BostonGene, Caris Life Sciences, Natera, Xilis, Palantir, Genentech. Consulting/stock ownership: Engine Biosciences, NaDeNo Nanoscience.Dan Zhao: Clinical trials with hMirati/BMS, Phanes, CARsgen, TriSalus and Affini-T; Consulting for Ipsen.
Figures
References
-
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. - PubMed
-
- Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23. - PubMed
-
- Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, Sudhakar N, Bowcut V, Baer BR, Ballard JA, et al. The KRAS < sup > G12C Inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-Mutant cancers in mouse models and patients. Cancer Discov. 2020;10(1):54–71. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

