KRAS mutation detection by liquid biopsy for pancreatic ductal adenocarcinoma
- PMID: 40247392
- PMCID: PMC12004821
- DOI: 10.1186/s13045-025-01696-0
KRAS mutation detection by liquid biopsy for pancreatic ductal adenocarcinoma
Abstract
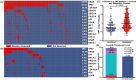
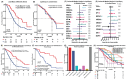
The clinical utility of liquid biopsy (LB) for pancreatic ductal adenocarcinoma (PDAC) remain understudied. Our single-institution cohort of 311 PDAC patients with non-tumor tissues informed LB found 81.2% positivity (N = 186) in metastatic cases and in 52.4% (N = 43) of localized disease. KRAS mutations were detected in 64.6% (N = 148) of metastatic cases and 16% (N = 13) for localized disease. Positive LB, especially KRAS mutation detection, is associated with worse overall survival (OS) in metastatic PDAC (median 14.5 vs. 31.3 months, HR = 2.7, 95%CI = 1.7-4.3, P < 0.0001). The positive concordance rates of KRAS and TP53 mutations were 63% and 68% in metastatic disease but only 7% (KRAS) and 33% (TP53) in localized disease, respectively. Among the 41 patients who underwent serial liquid biopsy testing, 25% tested positive after an initial negative result. LB detects therapeutically targetable mutations in 58.5% of PDAC patients and is associated with OS.
Keywords: KRAS; Liquid biopsy; Molecular profiling; Mutation; OS; PDAC; Pancreatic cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the MD Anderson Institutional Review Board (IRB), protocol number 2023-0091. A waiver of informed consent was granted per the USA federal regulation 45 CFR 46.116(f) for this retrospective study. Competing interests: Brandon Smaglo: Consulting for Ipsen.Shubham Pant: Advisory for Zymeworks, Ipsen, Novartis, Janssen, Boehringer Ingelheim, and AskGene Pharma; and he receives research funding from Mirati Therapeutics, Lilly, Xencor, Novartis, Rgenix, Bristol-Myers Squibb, Astellas Pharma, Framewave, 4D Phar-ma, Boehringer Ingelheim, NGM Biopharmaceuticals, Janssen, Arcus Biosciences, Elicio Therapeutics, Bionte, Ipsen, Zymeworks, Pfizer, ImmunoMET, Imuneering, and Amal Therapeutics. Anirban Maitra: Consultant for Tezcat Biosciences is listed as an inventor of a patent licensed to Thrive Earlier Detection (an Exact Sciences Company) relevant to early detection of pancreatic cancer. John Paul Shen: Grant/research support/collaboration: Celsius Therapeutics, BostonGene, Caris Life Sciences, Natera, Xilis, Palantir, Genentech. Consulting/stock ownership: Engine Biosciences, NaDeNo Nanoscience.Dan Zhao: Clinical trials with hMirati/BMS, Phanes, CARsgen, TriSalus and Affini-T; Consulting for Ipsen.
Figures
References
-
- Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, Sudhakar N, Bowcut V, Baer BR, Ballard JA, et al. The KRAS < sup > G12C Inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-Mutant cancers in mouse models and patients. Cancer Discov. 2020;10(1):54–71. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

