Sustained tenascin-C expression drives neointimal hyperplasia and promotes aortocaval fistula failure
- PMID: 40247455
- PMCID: PMC12150301
- DOI: 10.1152/ajpheart.00661.2024
Sustained tenascin-C expression drives neointimal hyperplasia and promotes aortocaval fistula failure
Abstract
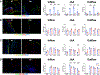
End-stage kidney disease (ESKD) impacts over 740,000 individuals in the United States, with many patients relying on arteriovenous fistulae (AVF) for hemodialysis due to superior patency and reduced infections. However, AVF patency is reduced by thrombosis and neointimal hyperplasia, yielding a 1-yr patency of only 40%-50%. We hypothesized that tenascin-C (TNC), a regulator of inflammation and immune responses after injury, also regulates venous remodeling during AVF maturation. AVF were created in wild-type (WT) and Tnc knockout (Tnc-/-) mice, and proteomic analyses were conducted to identify protein changes between sham and AVF WT tissue. Immunofluorescence and Western blot assays compared venous tissue from WT and Tnc-/- mice. In vitro studies using human umbilical vein endothelial cells and human umbilical vein smooth muscle cells examined TNC-siRNA effects on thrombomodulin (THBD) and NF-κB. Macrophages from WT and Tnc-/- mice were assessed for anti-inflammatory phenotype polarization and tissue factor expression. TNC expression was spatially and temporally regulated in WT mice with AVF, and TNC colocalized with matrix remodeling but not with THBD expression; TNC expression was downregulated in patent AVF but sustained in occluded AVF, both in WT mice and human AVF specimens. Tnc-/- mice had reduced AVF patency, less wall thickening, and increased thrombosis, with increased THBD expression. In vitro, TNC-siRNA increased THBD and reduced NF-κB activation. Macrophages from Tnc-/- mice showed increased anti-inflammatory macrophage polarization and tissue factor expression, facilitating thrombosis. Sustained TNC expression drives neointimal hyperplasia and AVF failure by promoting a prothrombotic, inflammatory microenvironment. Targeting TNC pathways may enhance AVF patency and improve dialysis outcomes.NEW & NOTEWORTHY This study identifies Tenascin-C (TNC) as a key regulator of arteriovenous fistula (AVF) patency. TNC is spatially and temporally regulated, driving neointimal hyperplasia and thrombosis by promoting a prothrombotic, inflammatory microenvironment. In Tnc-/- mice, reduced TNC expression increased thrombomodulin and anti-inflammatory macrophage polarization but impaired wall thickening and AVF patency. These findings link sustained TNC expression to AVF failure and suggest that targeting TNC pathways could enhance AVF outcomes in patients requiring hemodialysis.
Keywords: arteriovenous fistulae; tenascin-C; thrombomodulin; thrombosis; venous remodeling.
Conflict of interest statement
Disclosure:
The authors have declared that no conflict of interest exists.
Figures



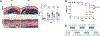


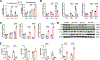
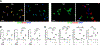
References
-
- Al-Jaishi AA, et al. , Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis, 2014. 63(3): p. 464–78. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

