Novel Foraging Mechanisms in Atypical Excavate Flagellates
- PMID: 40247630
- PMCID: PMC12006737
- DOI: 10.1111/jeu.70010
Novel Foraging Mechanisms in Atypical Excavate Flagellates
Abstract
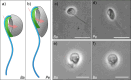
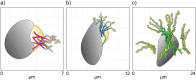
Most excavates, a paraphyletic assemblage of flagellates, typically present an active vaned flagellum that drives a feeding current through a ventral groove for predation. However, some have "atypical" morphologies. Here, we describe the foraging mechanisms in heteroloboseid flagellates (Discoba) that have a groove but lack the seemingly crucial vane. The percolomonads barbeliid AE-1 and Percolomonas doradorae form a functional vane with four adjacent flagella undulating with lateral asymmetry, creating an erratic flow that rapidly "sucks" water into the groove and expels it on the other side. This flow attenuates rapidly away from the cell, consistent with the flagellar pump acting as an instantaneous point sink. Conversely, Pharyngomonas kirbyi generates a continuous flow through the groove with two asynchronously moving posterior flagella. Despite the unexplained fluid dynamics, this flow has a further reach, consistent with describing the flagellar pump as a point force (stokeslet). While the volumetric flow rate through the groove-a measure of the maximum potential clearance rate-of the two percolomonads is similar to clearance rates estimated for other phagotrophic flagellates, it is an order of magnitude lower for Ph. kirbyi, which may afford lower rates due to high prey concentration in its hypersaline environment.
Keywords: clearance rate; feeding current; heteroloboseids; prey capture; vaneless flagella; ventral feeding groove.
© 2025 The Author(s). Journal of Eukaryotic Microbiology published by Wiley Periodicals LLC on behalf of International Society of Protistologists.
Figures

References
-
- Asadzadeh, S. S. , Walther J. H., Andersen A., and Kiørboe T.. 2022. “Hydrodynamic Interactions Are Key in Thrust‐Generation of Hairy Flagella.” Physical Review Fluids 7: 073101. 10.1103/PhysRevFluids.7.073101. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

