Protocol for differentiating primary human small airway epithelial cells at the air-liquid interface
- PMID: 40247673
- PMCID: PMC12169842
- DOI: 10.1152/ajplung.00380.2024
Protocol for differentiating primary human small airway epithelial cells at the air-liquid interface
Abstract
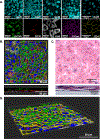
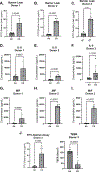
The air-liquid interface (ALI) culture is an important tool in pulmonary research as it models the physiological lung where the epithelium is apically exposed to air and basally to the endothelium and interstitium. Although there is an abundance of research that uses primary human bronchial epithelial cells (HBECs) to study larger airways, small airway epithelial cells (SAECs) are an untapped resource in comparison. Primary SAECs are a valuable cell population as they enable the study of pathologies in the bronchioles and are also a favorable surrogate for primary alveolar epithelial cells, which are invasive to collect from patients. Currently, there are limited resources on how to culture and differentiate SAECs at the ALI. Here, we provide an optimized, detailed protocol to address this knowledge gap. Key culture conditions that determine the quality and uniformity of differentiated SAECs include cell passage number, pH changes caused by media exhaustion and incubator CO2, seeding density, and collagen coating of the expansion flask and inserts. We also describe a FITC-dextran permeability assay to measure SAEC barrier integrity both as a pretest to select uniform wells with strong barrier integrity before an experiment and as a post-test to evaluate treatment effects afterward. The utility of the differentiated SAEC ALI model to ask biologically relevant questions is demonstrated by increased cytokine (IL-8, MIF, and CXCL-10) production and/or epithelial damage following exposure to cigarette smoke, lipopolysaccharide (LPS) or poly(I:C).NEW & NOTEWORTHY SAECs are not commonly used in pulmonary research, and this is reflected in a lack of literature on both SAEC primary research and methodological reports. Primary SAECs are an important resource as they enable the study of the small airways, which are implicated in a variety of pulmonary diseases, including chronic obstructive pulmonary disease (COPD). The detailed protocol described here bridges the knowledge gap on how to successfully differentiate primary human SAECs at the ALI.
Keywords: air-liquid interface; differentiation; methods; primary human cells; small airway epithelial cells.
Conflict of interest statement
DISCLOSURES
No conflicts of interest to disclose.
Figures




References
-
- Doni Jayavelu N, Altman MC, Benson B, Dufort MJ, Vanderwall ER, Rich LM, White MP, Becker PM, Togias A, Jackson DJ, Debley JS. Type 2 inflammation reduces SARS-CoV-2 replication in the airway epithelium in allergic asthma through functional alteration of ciliated epithelial cells. J Allergy Clin Immunol 152: 56–67, 2023. doi: 10.1016/J.JACI.2023.03.021. - DOI - PMC - PubMed
-
- Bulcaen M, Kortleven P, Liu RB, Maule G, Dreano E, Kelly M, Ensinck MM, Thierie S, Smits M, Ciciani M, Hatton A, Chevalier B, Ramalho AS, Casadevall i Solvas X, Debyser Z, Vermeulen F, Gijsbers R, Sermet-Gaudelus I, Cereseto A, Carlon MS. Prime editing functionally corrects cystic fibrosis-causing CFTR mutations in human organoids and airway epithelial cells. Cell Rep Med 5: 101544, 2024. doi: 10.1016/J.XCRM.2024.101544. - DOI - PMC - PubMed
-
- Wasnick R, Korfei M, Piskulak K, Henneke I, Wilhelm J, Mahavadi P, Dartsch RC, von der Beck D, Koch M, Shalashova I, Weiss A, Klymenko O, Askevold I, Fink L, Witt H, Hackstein H, El Agha E, Bellusci S, Klepetko W, Königshoff M, Eickelberg O, Schermuly RT, Braun T, Seeger W, Ruppert C, Guenther A. Notch1 Induces Defective Epithelial Surfactant Processing and Pulmonary Fibrosis. Am J Respir Crit Care Med 207: 283–299, 2023. doi: 10.1164/RCCM.202105-1284OC. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

