Evaluating the efficacy of 8Spheres microsphere embolization combined with iodine‑125 seed implantation in advanced refractory lung cancer: A retrospective study
- PMID: 40247985
- PMCID: PMC12004036
- DOI: 10.3892/ol.2025.15031
Evaluating the efficacy of 8Spheres microsphere embolization combined with iodine‑125 seed implantation in advanced refractory lung cancer: A retrospective study
Abstract
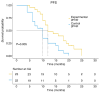
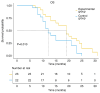
Patients with advanced non-small cell lung cancer (NSCLC) have seen improvements in care; however, outcomes remain poor for certain individuals despite treatment with radiation, chemotherapy, targeted therapies and immunotherapy. The present study aimed to assess the safety and efficacy of combining 8Spheres microsphere embolization with iodine-125 seed implantation for treating advanced refractory NSCLC. The retrospective analysis included 45 patients with advanced refractory NSCLC. Using the surv_cutpoint function in R, the optimal maximum tumor diameter threshold was determined as 53 mm, dividing patients into two groups: ≤53 mm and >53 mm. The study evaluated the association between treatment regimen, tumor diameter, and progression-free survival (PFS) and overall survival (OS). The findings demonstrated that the experimental group achieved a significantly longer median PFS (12 vs. 10 months; P=0.006) and OS (19 vs. 12 months; P=0.032) compared with the control group. Both the treatment approach and tumor size were identified as independent factors influencing survival. The risk of death was 2.291-fold higher for patients on the control regimen than for those in the experimental group. Similarly, patients with a tumor diameter of >53 mm had a 2.723-fold higher risk of death than those with a tumor diameter of ≤53 mm. Adverse events were mild and resolved in both groups. In summary, the combination of 8Spheres microsphere embolization and iodine-125 seed implantation demonstrate promising clinical outcomes and it may be a viable treatment for advanced refractory NSCLC. Additionally, maximum tumor diameter was strongly associated with patient survival and therefore it may serve as a valuable prognostic indicator to guide treatment decisions.
Keywords: 8Spheres microspheres; NSCLC; bronchial artery infusion chemotherapy embolization; iodine-125 seeds.
Copyright: © 2025 Ran et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Remon J, Soria JC, Peters S, ESMO Guidelines Committee. Electronic address clinicalguidelines@esmo.org: Early and locally advanced non-small-cell lung cancer: An update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32:1637–1642. doi: 10.1016/j.annonc.2021.08.1994. - DOI - PubMed
-
- Zhao YW, Liu S, Qin H, Sun JB, Su M, Yu GJ, Zhou J, Gao F, Wang RY, Zhao T, Zhao GS. Efficacy and safety of CalliSpheres drug-eluting beads for bronchial arterial chemoembolization for refractory non-small-cell lung cancer and its impact on quality of life: A multicenter prospective study. Front Oncol. 2023;13:1110917. doi: 10.3389/fonc.2023.1110917. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
