Amoxicillin high-dose dual therapy for Helicobacter pylori primary eradication: Proton pump inhibitor and potassium-competitive acid blocker, which's better?
- PMID: 40248055
- PMCID: PMC12001176
- DOI: 10.3748/wjg.v31.i13.100863
Amoxicillin high-dose dual therapy for Helicobacter pylori primary eradication: Proton pump inhibitor and potassium-competitive acid blocker, which's better?
Abstract
Background: Effective acid suppression significantly enhances the eradication rate of Helicobacter pylori (H. pylori).
Aim: To assess the efficacy and safety of high-dose dual therapy (HDDT) utilizing various highly potent antisecretory medications, thereby providing additional clinical guidance for H. pylori eradication.
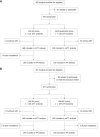
Methods: The study population comprised untreated H. pylori patients from three medical centers in central China. From February 10, 2024 to March 31, 2024, 439 subjects were randomly allocated to either the esomeprazole-amoxicillin (EA) or esomeprazole-amoxicillin-clarithromycin-bismuth (B-quadruple) group. Subsequently, from April 1, 2024 to May 10, 2024, 367 subjects were randomly assigned to either the vonoprazan-amoxicillin (VA) or vonoprazan-amoxicillin-clarithromycin (VAC) group. The study recorded treatment efficacy, adverse events, compliance, symptom alleviation, and associated costs.
Results: EA-dual demonstrated non-inferiority to B-quadruple regimen in modified intention-to-treat (mITT) and per-protocol (PP) analyses (P < 0.025). However, the eradication rate of EA was lower than that of the B-quadruple group [70.59% vs 83.49%, 92.86% vs 98.38%, 93.94% vs 98.38%, intention-to-treat (ITT), mITT, PP respectively, P < 0.05]. In ITT, mITT, and PP analyses, VA-dual was non-inferior to VAC treatment (84.15% vs 83.15%, 96.25% vs 92.73%, 96.75% vs 93.75%, P < 0.025). No significant differences were observed in adverse events, compliance, and symptom relief between groups. VA exhibited the lowest cost. Antibiotic use within 2 years, poor compliance, and suburban residence were associated with reduced eradication efficacy (P < 0.05).
Conclusion: The HDDT based on vonoprazan demonstrated non-inferiority to the VAC triple regimen, suggesting its potential as a recommended first-line treatment for H. pylori eradication. While B-quadruple therapy showed better eradication rate than EA therapy, the latter proved non-inferior in mITT and PP analyses. Notably, antibiotic use within the preceding two years, adherence to treatment protocols, and patient residence emerged as critical factors influencing eradication success.
Keywords: Eradication rate; Esomeprazole; Helicobacter pylori; High-dose dual therapy; Vonoprazan.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflicts of interest.
Figures
References
-
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. - PubMed
-
- Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:553–564. - PubMed
-
- Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613–624. - PubMed
-
- Mülder DT, Hahn AI, Huang RJ, Zhou MJ, Blake B, Omofuma O, Murphy JD, Gutiérrez-Torres DS, Zauber AG, O'Mahony JF, Camargo MC, Ladabaum U, Yeh JM, Hur C, Lansdorp-Vogelaar I, Meester R, Laszkowska M. Prevalence of Gastric Precursor Lesions in Countries With Differential Gastric Cancer Burden: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2024;22:1605–1617.e46. - PMC - PubMed
-
- Piazuelo MB, Bravo LE, Mera RM, Camargo MC, Bravo JC, Delgado AG, Washington MK, Rosero A, Garcia LS, Realpe JL, Cifuentes SP, Morgan DR, Peek RM Jr, Correa P, Wilson KT. The Colombian Chemoprevention Trial: 20-Year Follow-Up of a Cohort of Patients With Gastric Precancerous Lesions. Gastroenterology. 2021;160:1106–1117.e3. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

