Decoding bronchopulmonary dysplasia in premature infants through an epigenetic lens
- PMID: 40248086
- PMCID: PMC12003331
- DOI: 10.3389/fmed.2025.1531169
Decoding bronchopulmonary dysplasia in premature infants through an epigenetic lens
Abstract
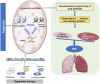
This review provides a comprehensive overview of the evolving insights into the epigenetic mechanisms associated with bronchopulmonary dysplasia (BPD). It specifically highlights the roles of DNA methylation, histone modifications, and RNA regulation in the development of BPD in premature infants. BPD results from complex interactions among genetic factors, environmental exposures, and neonatal stressors. Key findings suggest that intrauterine hypoxia, hyperoxia, and nutrition can lead to epigenetic alterations, affecting gene expression and methylation, which may serve as biomarkers for early BPD detection. RUNX3 is identified as a critical transcription factor influencing lung development and inflammation, while changes in DNA methylation and histone dynamics in cord blood are linked to immune dysregulation associated with BPD. The role of m6A RNA methylation regulators from the IGF2BP family affects mRNA stability and gene expression relevant to BPD. Additionally, specific histones and microRNAs, particularly from the miR-17∼92 cluster, are implicated in pulmonary development and vascular regulation. Long non-coding RNAs (lncRNAs), such as MALAT1, also play a role in gene regulation via competitive endogenous RNA networks, indicating their potential as biomarkers and therapeutic targets. The interplay of these epigenetic mechanisms underscores the need for further research to develop targeted interventions aimed at reducing BPD severity and enhancing health outcomes for at-risk neonates.
Keywords: DNA methylation; RUNX3; bronchopulmonary dysplasia; epigenetics; long non-coding RNAs; microRNAs.
Copyright © 2025 Dastgheib, Bahrami, Golshan-Tafti, Danaei, Azizi, Shahbazi, Yeganegi, Shiri, Masoudi and Neamatzadeh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Of mice and men: correlations between microRNA-17∼92 cluster expression and promoter methylation in severe bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol. 2016 Nov 1;311(5):L981-L984. doi: 10.1152/ajplung.00390.2016. Epub 2016 Sep 30. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27694474 Free PMC article.
-
Hyperoxia-induced methylation decreases RUNX3 in a newborn rat model of bronchopulmonary dysplasia.Respir Res. 2015 Jun 24;16(1):75. doi: 10.1186/s12931-015-0239-x. Respir Res. 2015. PMID: 26104385 Free PMC article.
-
Long non-coding RNA MALAT1 protects preterm infants with bronchopulmonary dysplasia by inhibiting cell apoptosis.BMC Pulm Med. 2017 Dec 13;17(1):199. doi: 10.1186/s12890-017-0524-1. BMC Pulm Med. 2017. PMID: 29237426 Free PMC article.
-
Epigenetic modifications in the development of bronchopulmonary dysplasia: a review.Pediatr Res. 2024 Aug;96(3):632-642. doi: 10.1038/s41390-024-03167-7. Epub 2024 Apr 3. Pediatr Res. 2024. PMID: 38570557 Review.
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
References
-
- Bahrami R, Golshan-Tafti M, Dastgheib S, Alijanpour K, Yeganegi M, Lookzadeh M, et al. A Comprehensive consolidation of data on the relationship between surfactant protein-B (SFTPB) polymorphisms and susceptibility to bronchopulmonary dysplasia. Fetal Pediatr Pathol. (2024) 43:436–54. 10.1080/15513815.2024.2400145 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

