Perilla frutescens Leaf-Derived Extracellular Vesicle-Like Particles Carry Pab-miR-396a-5p to Alleviate Psoriasis by Modulating IL-17 Signaling
- PMID: 40248109
- PMCID: PMC12003952
- DOI: 10.34133/research.0675
Perilla frutescens Leaf-Derived Extracellular Vesicle-Like Particles Carry Pab-miR-396a-5p to Alleviate Psoriasis by Modulating IL-17 Signaling
Abstract
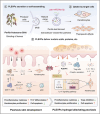
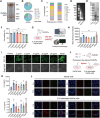
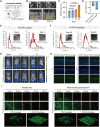
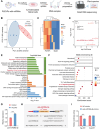
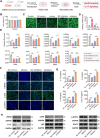
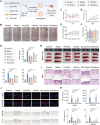
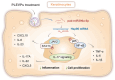
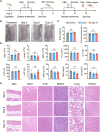
Psoriasis, a chronic inflammatory skin disorder, remains challenging to treat due to poor skin barrier penetration, limited efficacy, and adverse effects of current therapies. Natural plant-derived extracellular vesicle-like particles (EVPs) have emerged as biocompatible carriers for bioactive molecules. Among various medicinal plants screened, Perilla frutescens leaf-derived EVPs (PLEVPs) exhibited strong anti-inflammatory and antioxidant effects. By incorporating PLEVPs into a hydrogel formulation, we enhanced their stability, retention at psoriatic lesions, and transdermal delivery efficiency. In vivo studies demonstrated that the PLEVPs markedly alleviated psoriasis symptoms in both preventive and therapeutic mouse models, outperforming conventional treatments. This effect was attributed to reduced oxidative stress, modulation of Treg cells, and promotion of keratinocyte apoptosis. Transcriptomic analysis revealed enrichment of the interleukin-17 (IL-17) signaling pathway, a major driver of psoriasis, while small RNA sequencing identified pab-miR396a-5p, an endogenous microRNA (miRNA) within PLEVPs, as a key regulator. Mechanistic studies showed that pab-miR396a-5p targets the 3'-untranslated region of plant heat shock protein 83a, a homolog of mammalian heat shock protein 90, leading to the suppression of nuclear factor-kappa B and Janus kinase/signal transducers and activators of transcription signaling, inhibiting the IL-17 signaling pathway. Validation using lipid nanoparticles encapsulating pab-miR396a-5p mimics confirmed comparable therapeutic effects. This study highlights the potential of plant-derived EVPs as carriers of endogenous miRNAs, enabling interkingdom communication and offering a scalable platform for psoriasis therapy.
Copyright © 2025 Yali Liu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Sewerin P, Brinks R, Schneider M, Haase I, Vordenbäumen S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2019;78(2):286–287. - PubMed
-
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis. JAMA. 2020;323(19):1945. - PubMed
-
- Dai A, Kim SJ. Systemic calcineurin inhibitors tacrolimus and voclosporin: A review of off-label dermatologic uses. J Am Acad Dermatol. 2024;90(2):358–367. - PubMed
-
- Evans M, Dahdaleh S, Karunaratne K, Pritchard J, Rinaldi S, Viegas S. 192 tacrolimus-induced chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): A case series. J Neurol Neurosurg Psychiatry. 2022;93:e2.
LinkOut - more resources
Full Text Sources

