Double-Blind, Randomized, Placebo-Controlled, Crossover Study of Oral Cannabidiol and Tetrahydrocannabinol for Essential Tremor
- PMID: 40248111
- PMCID: PMC12005140
- DOI: 10.5334/tohm.1005
Double-Blind, Randomized, Placebo-Controlled, Crossover Study of Oral Cannabidiol and Tetrahydrocannabinol for Essential Tremor
Abstract
Background: Essential tremor (ET) is characterized by often disabling action tremors. No pharmacological agent has been developed specifically for symptomatic treatment. Anecdotal reports describe tremor improvement with cannabis, but no evidence exists to support these claims. We conducted a phase Ib/II double-blind, placebo-controlled, crossover pilot trial in participants with ET to investigate tolerability, safety, and efficacy of Tilray TN-CT120 LM, an oral pharmaceutical-grade formulation containing tetrahydrocannabinol (THC) 5 mg and cannabidiol (CBD) 100 mg. Our objectives were to determine if short-term THC/CBD exposure improved tremor amplitude and was tolerated.
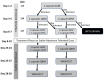
Methods: Participants with ET were randomized (1:1) to receive either TN-CT120 LM or placebo. Dose titration, driven by tolerability, was attempted every 2-3 days to three capsules daily maximum. Participants remained on the highest tolerated dose for two weeks before returning to complete assessments. After completing the first arm, participants titrated off the agent, underwent a three-week washout, and then returned for the same procedures with the alternate compound. The primary endpoint was tremor amplitude change from baseline using digital spiral assessment. Secondary endpoints explored safety and tolerability.
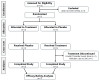
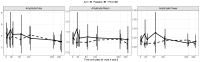
Results: Among thirteen participants screened, seven were eligible and enrolled. Five completed all visits; one withdrew following a serious adverse event, and another did not tolerate the lowest dose. Intent-to-treat analyses performed for six participants did not reveal significant effects on primary or secondary endpoints.
Conclusions: This pilot trial did not detect any signals of efficacy of THC/CBD in ET. Although preliminary due to the small sample size, our data do not support anecdotal reports of cannabinoid effectiveness for ET.
Highlights: This double-blind, randomized, placebo-controlled efficacy and tolerability pilot trial did not detect any signals of efficacy of oral cannabidiol and tetrahydrocannabinol in reducing essential tremor amplitude using either digital outcome measures or clinical rating scales. The oral cannabinoids were well-tolerated by most (five out of seven) participants.
Keywords: cannabis, therapeutics; digital outcome measures; essential tremor.
Copyright: © 2025 The Author(s).
Conflict of interest statement
K. Longardner has received grant support from an institutional KL2 grant 1KL2TR001444 and NIH grant R21 NS114764-01A1. She has served as consultant to Abbvie, Boston Scientific, and Prime. Q. Shen reports no disclosures. F.X. Castellanos conducted an open RCT of cannabidiol provided by Jazz Pharmaceuticals; he received support from NIH grants R33MH113663 and U01DK140791; and he is chief field editor of Frontiers in Neuroimaging. B. Tan reports no disclosures. R. Gandhi reports no disclosures. B.A. Wright reports no disclosures. J. Momper reports no disclosures. F.B. Nahab serves as an employee of Neuron23. He has also served as consultant to ActualSignal, Attune Neurosciences, and Encora.
Figures