When the conduction disturbance expresses a cardiomyopathy
- PMID: 40248293
- PMCID: PMC12001799
- DOI: 10.1093/eurheartjsupp/suaf014
When the conduction disturbance expresses a cardiomyopathy
Abstract
Electrocardiogram may play a crucial role in the diagnostic workup of different cardiomyopathies. Electrocardiogram abnormalities in impulse generation and transmission may be an early marker of these insidious pathologies. Some findings are suggestive of definite disorders, and other findings are less sensitive and/or specific, but may orient towards a specific diagnosis in a patient with a peculiar phenotype. Electrocardiogram findings not only could help to early recognize affected patients but may also have an important prognostic role in evaluating disease evolution over time and identifying patients at higher risk of sudden cardiac death. Electrocardiogram reading and the careful interpretation of its features remain a cornerstone for orienting the diagnosis towards specific forms and provide useful tools for risk stratification.
Keywords: Arrhythmogenic cardiomyopathy; Cardiomyopathy; Conduction disturbances; Electrocardiogram.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures
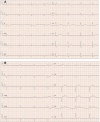

References
-
- Elizari MV. The normal variants in the left bundle branch system. J Electrocardiol 2017;50:389–399. - PubMed
-
- Finocchiaro G, Merlo M, Sheikh N, De Angelis G, Papadakis M, Olivotto I et al. The electrocardiogram in the diagnosis and management of patients with dilated cardiomyopathy. Eur J Heart Fail 2020;22:1097–1107. - PubMed
-
- van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers: a European cohort study. J Am Coll Cardiol 2012;59:493–500. - PubMed
LinkOut - more resources
Full Text Sources
