Engineering Pseudomonas putida for production of 3-hydroxyacids using hybrid type I polyketide synthases
- PMID: 40248344
- PMCID: PMC12005932
- DOI: 10.1016/j.mec.2025.e00261
Engineering Pseudomonas putida for production of 3-hydroxyacids using hybrid type I polyketide synthases
Abstract
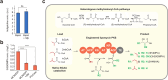
Engineered type I polyketide synthases (T1PKSs) are a potentially transformative platform for the biosynthesis of small molecules. Due to their modular nature, T1PKSs can be rationally designed to produce a wide range of bulk or specialty chemicals. While heterologous PKS expression is best studied in microbes of the genus Streptomyces, recent studies have focused on the exploration of non-native PKS hosts. The biotechnological production of chemicals in fast growing and industrial relevant hosts has numerous economic and logistic advantages. With its native ability to utilize alternative feedstocks, Pseudomonas putida has emerged as a promising workhorse for the sustainable production of small molecules. Here, we outline the assessment of P. putida as a host for the expression of engineered T1PKSs and production of 3-hydroxyacids. After establishing the functional expression of an engineered T1PKS, we successfully expanded and increased the pool of available acyl-CoAs needed for the synthesis of polyketides using transposon sequencing and protein degradation tagging. This work demonstrates the potential of T1PKSs in P. putida as a production platform for the sustainable biosynthesis of unnatural polyketides.
Keywords: 3-Hydroxyacid production; Polyketide synthase engineering; Protease degradation tag; Pseudomonas putida; Transposon sequencing.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jay D. Keasling has financial interests in Ansa Biotechnologies, Apertor Pharma, Berkeley Yeast, Demetrix, Lygos, Napigen, ResVita Bio, and Zero Acre Farms.
Figures



Similar articles
-
Maximizing Heterologous Expression of Engineered Type I Polyketide Synthases: Investigating Codon Optimization Strategies.ACS Synth Biol. 2023 Nov 17;12(11):3366-3380. doi: 10.1021/acssynbio.3c00367. Epub 2023 Oct 18. ACS Synth Biol. 2023. PMID: 37851920 Free PMC article.
-
Stepwise genetic engineering of Pseudomonas putida enables robust heterologous production of prodigiosin and glidobactin A.Metab Eng. 2021 Sep;67:112-124. doi: 10.1016/j.ymben.2021.06.004. Epub 2021 Jun 24. Metab Eng. 2021. PMID: 34175462 Free PMC article.
-
Unraveling the iterative type I polyketide synthases hidden in Streptomyces.Proc Natl Acad Sci U S A. 2020 Apr 14;117(15):8449-8454. doi: 10.1073/pnas.1917664117. Epub 2020 Mar 26. Proc Natl Acad Sci U S A. 2020. PMID: 32217738 Free PMC article.
-
Engineered polyketides: Synergy between protein and host level engineering.Synth Syst Biotechnol. 2017 Sep 7;2(3):147-166. doi: 10.1016/j.synbio.2017.08.005. eCollection 2017 Sep. Synth Syst Biotechnol. 2017. PMID: 29318196 Free PMC article. Review.
-
Herboxidiene biosynthesis, production, and structural modifications: prospect for hybrids with related polyketide.Appl Microbiol Biotechnol. 2015 Oct;99(20):8351-62. doi: 10.1007/s00253-015-6860-2. Epub 2015 Aug 19. Appl Microbiol Biotechnol. 2015. PMID: 26286508 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

