Limitations of human t-lymphotropic virus type 1 antibody testing in hospitals of endemic regions in China
- PMID: 40248369
- PMCID: PMC12003307
- DOI: 10.3389/fcimb.2025.1474526
Limitations of human t-lymphotropic virus type 1 antibody testing in hospitals of endemic regions in China
Abstract
Purpose: This study aims to evaluate the sensitivity and specificity of human T-lymphotropic virus type 1 (HTLV-1) antibody testing within hospitals located in HTLV-1 endemic areas of China.
Method: We performed a retrospective analysis of the clinical records and laboratory results for 1,147 patients who underwent HTLV-1 antibody testing using the Wantai HTLV-1 antibody detection kit and Polymerase Chain Reaction (PCR) testing for HTLV-1 nucleic acids, at Fujian Medical University Union Hospital between 2017 and 2023.
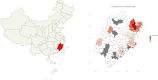
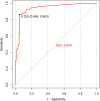
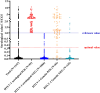
Result: The study population comprised 674 males (58.8%) and 473 females (41.2%), with an age distribution ranging from 7 to 86 years, and a median age of 50 years. Of the patients, 81 (7.1%) tested positive for HTLV-1 antibodies, including 39 males and 42 females. Predominantly, these positive cases were identified within the hematology department (93.8%). The cases originated from several high-prevalence coastal regions in Fujian province, such as Pingtan Island, Fuqing, Changle, Lianjiang, Fuan, Shouning, Xiapu, Zhouning, Fuding, Jiaocheng, Xiuyu, and Licheng. According to current standards for interpreting positive results, only 79.6% of patients with adult T-cell leukemia/lymphoma (ATLL) confirmed by HTLV-1 nucleic acid testing presented positive antibody results. Comparison of HTLV-1 antibody and nucleic acid test results revealed that the antibody test possessed a sensitivity of 63.0% and a specificity of 94.8%. A receiver operating characteristic (ROC) curve analysis determined that a threshold of 0.335 signal-to-cutoff (S/CO) was optimal for classifying positive antibody test results, yielding a sensitivity of 86.3% and a specificity of 94.4%.
Conclusion: The Wantai HTLV-1 antibody test kit, when utilized in hospitals within endemic regions, exhibits a high level of specificity. However, its sensitivity is found to be lacking when evaluated against the current standards for the interpretation of positive results. For patients with a high clinical suspicion of HTLV-1 infection-related diseases, it is crucial to conduct testing of HTLV-1 antibodies and nucleic acids.
Keywords: antibodies; epidemiology; hematology; human T-lymphotropic virus type 1; nucleic acid testing.
Copyright © 2025 Chen, Chen, Zheng, Yang, Wu, Hu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
[Serological survey of human T lymphotropic virus type I (HTLV-1) IgG antibody in south region of Xinjiang].Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1997 Dec;11(4):366-8. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1997. PMID: 15617250 Chinese.
-
[Sero-epidemiological study on the human T-cell leukaemia virus type I/II infection in the east coastal areas of Fujian province].Zhonghua Liu Xing Bing Xue Za Zhi. 2004 May;25(5):428-30. Zhonghua Liu Xing Bing Xue Za Zhi. 2004. PMID: 15231172 Chinese.
-
Epidemiology of anti-human T-cell leukemia virus type I antibody and characteristics of adult T-cell leukemia in China.Chin Med J (Engl). 1995 Dec;108(12):902-6. Chin Med J (Engl). 1995. PMID: 8728941
-
Seroprevalence of human T-lymphotropic virus infection among blood donors in China: a first nationwide survey.Retrovirology. 2021 Jan 7;18(1):2. doi: 10.1186/s12977-020-00546-w. Retrovirology. 2021. PMID: 33413457 Free PMC article.
-
[Human retrovirus HTLV-1: descriptive and molecular epidemiology, origin, evolution, diagnosis and associated diseases].Bull Soc Pathol Exot. 2011 Aug;104(3):167-80. doi: 10.1007/s13149-011-0174-4. Epub 2011 Jul 27. Bull Soc Pathol Exot. 2011. PMID: 21796326 Review. French.
References
-
- Canavaggio M., Leckie G., Allain J. P., Steaffens J. W., Laurian Y., Brettler D., et al. . (1990). The prevalence of antibody to HTLV-I/II in United States plasma donors and in United States and French hemophiliacs. Transfusion. 30, 780–782. doi: 10.1046/j.1537-2995.1990.30991048781.x - DOI - PubMed
-
- Chen X., Liu F., Fu X., Feng Y., Zhang D., Liu H., et al. . (2019). Prevalence of human T-cell lymphotropic virus type-1 infection among blood donors in mainland China: a systematic review and meta-analysis of the last 20 years. Expert Rev. Hematol. 12, 579–587. doi: 10.1080/17474086.2019.1632703 - DOI - PubMed
-
- Chen Y., Lv L., Ye Y., Li J., Huang M., Hu J., et al. . (1992). Research on the morphology, immunology, cytogenetics, and clinical aspects of adult T-cell leukemia. Chin. J. Hematology. 08, 110.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

