Integrating TSPO-PET imaging with metabolomics for enhanced prognostic accuracy in multiple sclerosis
- PMID: 40248672
- PMCID: PMC12004482
- DOI: 10.1136/bmjno-2025-001026
Integrating TSPO-PET imaging with metabolomics for enhanced prognostic accuracy in multiple sclerosis
Abstract
Background: Predicting disease progression in multiple sclerosis (MS) remains challenging. PET imaging with 18 kDa translocator protein (TSPO) radioligands can detect microglial and astrocyte activation beyond MRI-visible lesions, which has been shown to be highly predictive of disease progression. We previously demonstrated that nuclear magnetic resonance (NMR)-based metabolomics could accurately distinguish between relapsing-remitting (RRMS) and secondary progressive MS (SPMS). This study investigates whether combining TSPO imaging with metabolomics enhances predictive accuracy in a similar setting.
Methods: Blood samples were collected from 87 MS patients undergoing PET imaging with the TSPO-binding radioligand 11C-PK11195 in Finland. Patient disability was assessed using the expanded disability status scale (EDSS) at baseline and 1 year later. Serum metabolomics was performed to identify biomarkers associated with TSPO binding and disease progression.
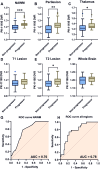
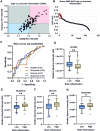
Results: Greater TSPO availability in the normal-appearing white matter and perilesional regions correlated with higher EDSS. Serum metabolites glutamate (p=0.02), glutamine (p=0.006), and glucose (p=0.008), detected by NMR, effectively distinguished future progressors. These three metabolites alone predicted progression with the same accuracy as TSPO-PET imaging (AUC 0.78; p=0.0001), validated in an independent cohort. Combining serum metabolite data with PET imaging significantly improved predictive power, achieving an AUC of 0.98 (p<0.0001).
Conclusion: Measuring three specific serum metabolites is as effective as TSPO imaging in predicting MS progression. However, integrating TSPO imaging with serum metabolite analysis substantially enhances predictive accuracy. Given the simplicity and affordability of NMR analysis, this approach could lead to more personalised, accessible treatment strategies and serve as a valuable tool for clinical trial stratification.
Keywords: MULTIPLE SCLEROSIS; PET.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
No, there are no competing interests.
Figures


References
-
- Cosenza-Nashat M, Zhao M-L, Suh H-S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35:306–28. doi: 10.1111/j.1365-2990.2008.01006.x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
