Closer proximity of pre-treatment CD4+ T cells to CD8+ T cells favor response to neoadjuvant immunotherapy in patients with PD-L1 low-expressing non-small cell lung cancer
- PMID: 40248717
- PMCID: PMC12000951
- DOI: 10.21037/tlcr-24-886
Closer proximity of pre-treatment CD4+ T cells to CD8+ T cells favor response to neoadjuvant immunotherapy in patients with PD-L1 low-expressing non-small cell lung cancer
Abstract
Background: Neoadjuvant chemo-immunotherapy improves non-small cell lung cancer (NSCLC) outcomes, but remission rates vary, emphasizing the need for biomarkers. This study aimed to investigate the impact of the baseline intratumoral CD4+ T-cell-adjacent microenvironment on the efficacy of neoadjuvant immunotherapy in NSCLC and its correlation with hypoxia-inducible factor-1α (HIF-1α), microvessel density (MVD), and cancer-associated fibroblasts (CAFs).
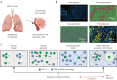
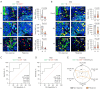
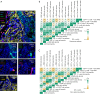
Methods: Tumor samples from 49 NSCLC patients before neoadjuvant immunotherapy were retrospectively collected and subjected to multiplex immunohistochemistry staining (panel 1: DAPI/CK/CD4/CD8/CD68; Panel 2: DAPI/CK/CD4/HIF-1α/CD31/α-SMA) to characterize CD4+ T cells, CD8+ T cells CD68+ macrophages, HIF-1α+ cells, HIF-1α+CD4+ cells, MVD, and CAF. Mann-Whitney U test and receiver operating characteristic (ROC) curve were used to assess the relationship between the number and spatial distribution of each metric and the efficacy of the treatment, and Spearman's rank correlation was used to assess the correlation of each metric.
Results: In 49 NSCLC patients, responders (54.2%) and non-responders (45.8%). Single-indicator analysis revealed a positive correlation between high infiltration of CD8+ T cells in the stromal area and response to treatment in overall and programmed cell death-ligand 1 (PD-L1) low-expressing patients [CD8+ T (str) density: overall patient, 38 vs. 16, P=0.03; tumor proportion score (TPS) 1-49% subgroup, 37 vs. 14, P=0.04], with an area under curve (AUC) 0.684 and 0.746, respectively. CD4+ T cells combined with CD8+ T cells or CD68+ macrophages were analyzed and found to be more efficacious than CD4+ThiCD8+Thi compared to CD4+TloCD8+Tlo in patients with low expression of PD-L1 (P=0.03). Assessment of the nearest neighbor distance (NND) of CD4+ T cells and their adjacent cells revealed that the closer the CD4+ T cells and CD8+ T cells in the overall compartment, the better the efficacy in NSCLC patients, especially in patients with low PD-L1 expression [CD4+ T to CD8+ T (all) NND: overall patients, 34 vs. 47 μm, P=0.03; TPS 1-49% subgroup, 34 vs. 69 μm, P=0.006], and the AUC was 0.670 and 0.830, respectively. Notably, this favorable spatial interaction may not be dependent on direct contact between CD4+ T cells and CD8+ T cells within 10/20/30 μm (P>0.05). Furthermore, in overall and PD-L1 low-expressing patients, the closer the distance between CD4+ T cells and CD8+ T cells, the higher the MVD (overall patients, r=-0.39, P=0.008; TPS 1-49% subgroup, r=-0.49, P=0.01).
Conclusions: The baseline intratumoral CD4+ T-cell-adjacent microenvironment in NSCLC is associated with the efficacy of neoadjuvant immunotherapy for NSCLC, with the closer proximity of pre-treatment CD4+ T cells and CD8+ T cells, the better the treatment efficacy in NSCLC patients (even especially in the low-expressing PD-L1 population), and is associated with high MVD.
Keywords: CD4+ T cells; Lung cancer; multiplex immunohistochemistry; neoadjuvant immunotherapy; spatial interaction.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-886/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Stromal PD-L1-Positive Regulatory T cells and PD-1-Positive CD8-Positive T cells Define the Response of Different Subsets of Non-Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy.J Thorac Oncol. 2018 Apr;13(4):521-532. doi: 10.1016/j.jtho.2017.11.132. Epub 2017 Dec 18. J Thorac Oncol. 2018. PMID: 29269008
-
Functional status and spatial interaction of T cell subsets driven by specific tumor microenvironment correlate with recurrence of non-small cell lung cancer.Front Immunol. 2023 Jan 4;13:1022638. doi: 10.3389/fimmu.2022.1022638. eCollection 2022. Front Immunol. 2023. PMID: 36685566 Free PMC article.
-
The Spatial Proximity of CD8+ FoxP3+PD-1+ Cells to Tumor Cells: A More Accurate Predictor of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer.Curr Oncol. 2025 Apr 30;32(5):262. doi: 10.3390/curroncol32050262. Curr Oncol. 2025. PMID: 40422521 Free PMC article.
-
The role of spatial interplay patterns between PD-L1-positive tumor cell and T cell in recurrence of locally advanced non-small cell lung cancer.Cancer Immunol Immunother. 2023 Jul;72(7):2015-2027. doi: 10.1007/s00262-023-03380-z. Epub 2023 Feb 4. Cancer Immunol Immunother. 2023. PMID: 36738309 Free PMC article.
-
Is neoadjuvant immunotherapy necessary in patients with programmed death ligand 1 expression-negative resectable non-small cell lung cancer? A systematic review and meta-analysis.Lung Cancer. 2024 May;191:107799. doi: 10.1016/j.lungcan.2024.107799. Epub 2024 Apr 23. Lung Cancer. 2024. PMID: 38669725
References
-
- Spicer J, Girard N, Provencio M, et al. Neoadjuvant nivolumab (NIVO)+ chemotherapy (chemo) vs chemo in patients (pts) with resectable NSCLC: 4-year update from Check-Mate 816. American Society of Clinical Oncology 2024;42:LBA8010.
-
- Carbone D, Waqar S, Chaft J, et al. 145MO Updated survival, efficacy and safety of adjuvant (adj) atezolizumab (atezo) after neoadjuvant (neoadj) atezo in the phase II LCMC3 study. J Thorac Oncol 2023;18:S90-S1.
-
- Deng H, Zhao Y, Cai X, et al. PD-L1 expression and Tumor mutation burden as Pathological response biomarkers of Neoadjuvant immunotherapy for Early-stage Non-small cell lung cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2022;170:103582. - PubMed
-
- Zhang F, Guo W, Zhou B, et al. Three-Year Follow-Up of Neoadjuvant Programmed Cell Death Protein-1 Inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2022;17:909-20. - PubMed
-
- Ricciuti B, Elkrief A, Alessi JVM, et al. Three-year outcomes and correlative analyses in patients with non–small cell lung cancer (NSCLC) and a very high PD-L1 tumor proportion score (TPS)≥ 90% treated with first-line pembrolizumab. American Society of Clinical Oncology;2022;40:9043. 10.1200/JCO.2022.40.16_suppl.9043 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
