Prognostic significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in second-line immunotherapy for patients with non-small cell lung cancer
- PMID: 40248735
- PMCID: PMC12000957
- DOI: 10.21037/tlcr-24-675
Prognostic significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in second-line immunotherapy for patients with non-small cell lung cancer
Abstract
Background: Immune checkpoint inhibitors remain a therapeutic option for chemotherapy pretreated patients with advanced non-small cell lung cancer (NSCLC). Given the lack of biomarkers, there is a need to look for predictive factors in this population. Inflammatory markers derived from peripheral blood cells (PBCs) may be a valuable diagnostic tool to assess the likelihood of clinical benefit. The aim of the study was to evaluate the efficacy of the treatment and to analyse the NLR and PLR predictive values.
Methods: Patients eligible for nivolumab or atezolizumab treatment in routine practice in two cancer centres between 2018 and 2021 were retrospectively analysed. Good performance status (ECOG 0-1), absence of EGFR, ALK, ROS1 alterations and no previous immune checkpoint inhibitors treatment were the inclusion criteria. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were calculated based on the results obtained before the start of immunotherapy. The median value was used as the cut-off point for comparative analyses.
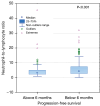
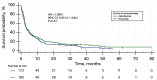
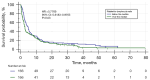
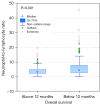
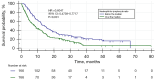
Results: The group of 332 patients was enrolled, 73.5% patients were in stage IV. The median NLR in the study group was 3.86±4.9 and the median PLR was 193.24±172.87. In the entire study group the disease control rate was 59 %, median PFS was 3.3 months [95% confidence interval (CI): 3.77 to 4.4], while median OS 11.57 months (95% CI: 9.03 to 12.73). In a univariate analysis the baseline values of NLR and PLR had a significant impact on survival, while age, gender, programmed death ligand 1 (PD-L1) expression, or type of treatment were not significant. In the multivariate Cox logistic regression model, a high value of NLR was the only factor that increased the risk of death [hazard ratio (HR) =1.6315, 95% CI: 1.2836 to 2.0737, P<0.001].
Conclusions: Inflammatory indices derived from peripheral blood cells-NLR and PLR-can help assess the prognosis of patients receiving immunotherapy. They also appear to be independent prognostic factors with regard to for PFS and OS.
Keywords: Neutrophil-to-lymphocyte ratio (NLR); immunotherapy; non-small cell lung cancer (NSCLC); platelet-to-lymphocyte ratio (PLR).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-675/coif). M.K.W. received consulting fees from BMS, MSD, Takeda as a member of the Advisory Boards, payment for lectures from BMS, MSD, Astra Zeneca, Takeda, ROCHE, travel grants from Astra Zeneca, Takeda, and MSD. P.K. received payments for lectures from Astra Zeneca, MSD, Pfizer, Johnson&Johnson, Roche, BMS, Amgen, and support for attending meetings and/or travel from Astra Zeneca, Roche, Johnson&Johnson, MSD, and Pfizer. P.K. also reports the participation in congresses and advisory boards for Astra Zeneca, Roche, MSD, Sanofi, and Pfizer. K.W.K. reports the Invited Lectures for BMS, MSD, Astra Zeneca. I.Ch received payments for lectures from BMS, MSD, Astra Zeneca, ROCHE; and travel grants from Astra Zeneca and MSD. T.J. received the payments for lectures from Astra Zeneca, MSD, BMS, Roche, Amgen, Takeda, Pfizer, Nutricia; and received support for attending meetings from MSD, Astra Zeneca, Takeda, Pfizer. M.K. received support for attending meetings from Roche, BMS, and Astra Zeneca. Advisory Boards BMS, MSD, ROCHE, Astra Zeneca. The other authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
