Safety and tolerability of conversion to siponimod from other disease-modifying therapies in patients with advancing forms of relapsing MS: Results from the EXCHANGE study
- PMID: 40248858
- PMCID: PMC12280243
- DOI: 10.1177/13524585251330085
Safety and tolerability of conversion to siponimod from other disease-modifying therapies in patients with advancing forms of relapsing MS: Results from the EXCHANGE study
Abstract
Background: Siponimod, a sphingosine-1-phosphate (S1P) receptor modulator, reduces relapses and delays disability progression in patients with active progressive multiple sclerosis (MS).
Objective: EXCHANGE assessed the safety/tolerability of siponimod in patients with advancing relapsing MS (RMS) converting from other disease-modifying therapies (DMTs).
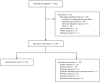
Methods: This 6-month, open-label, multicenter, single-arm, phase 3b study (NCT03623243) enrolled 185 patients with advancing RMS previously treated with other DMTs for ⩾3 months. Patients were converted to siponimod via a 6-day dose-titration regimen, or converted immediately, depending on prior DMT use.
Results: Treatment-related adverse events (AEs) were reported by 31.9% (59/185) of patients, with headache (8.1%, n = 15), dizziness (3.8%, n = 7), and nausea (3.2%, n = 6) most commonly reported. Overall, an increase in heart rate (HR) 6 hours following the first dose of siponimod was observed (+2.47 bpm [0.66; 4.29]; p = 0.008). Patients switching from fingolimod without dose titration experienced no change in HR. Serious AEs were reported by 4.9% (9/185) of patients, and 8.6% (16/185) of patients discontinued the study treatment due to AEs.
Conclusion: Conversion to siponimod from other DMTs was found to be generally well tolerated. Patients switching from other S1P-receptor modulators may be able to immediately transition to the siponimod maintenance dose without effects on HR.
Clinical trial registration: ClinicalTrials.gov: NCT03623243 (https://clinicaltrials.gov/study/NCT03623243).
Keywords: Bradycardia; S1P modulator; multiple sclerosis; safety; secondary progressive multiple sclerosis; siponimod; treatment satisfaction.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.J.F. has received personal consulting fees from AB Science, Biogen, Bristol Myers Squibb, Eli Lilly and Company, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Immunic, iNmune Bio, Janssen, Novartis, Sanofi, Siemens, and TG Therapeutics; has served on advisory committees for AB Science, Biogen, Immunic, Janssen, Novartis, and Sanofi; and has received clinical trial contract and research grant funding from Biogen, Novartis, and Sanofi. S.C. has received speaker fees from Biogen, Bristol Myers Squibb, Novartis, Roche/Genentech, and Sanofi-Genzyme; has served on advisory boards or as a consultant for Biogen, Bristol Myers Squibb, EMD Serono, Novartis, and Sanofi-Genzyme; and has received institutional (the Providence Brain and Spine Institute) research support from AbbVie, Adamas, Biogen, EMD Serono, MedDay, Novartis, Roche/Genentech, Sage Bionetworks, and Sanofi-Genzyme. Y.M-.D. has served as a consultant for and/or received grant support from: Acorda, Bayer, Biogen, Celgene/Bristol Myers Squibb, EMD Serono, Horizon, Janssen, Novartis, Questor, Roche/Genentech, Sanofi-Genzyme, TG Therapeutics, and Teva and has received grants from Chugai, the NIH NIAID Autoimmune Center of Excellence (UM1-AI110557-05, UM1 AI144298-01), Novartis, PCORI, and Sanofi-Genzyme. B.W-.G. has received consulting fees from Biogen, Bristol Myers Squibb, EMD Serono, Immunex, and SANA and has received research support from Biogen, Bristol Myers Squibb, EMD Serono, Genentech, and Novartis. L-.A.C. and G.M.C. are employees and stockholders of Novartis. S.A. is an employee and stockholder of Novartis AG. A.B-.O. has received personal fees for advisory board participation and/or consulting from Abata, Accure, Atara, Biogen, Bristol Myers Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Horizon, Immunic, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, Sangamo, Sanofi-Genzyme, and Viracta; and has received grant support to the University of Pennsylvania from Biogen, Merck/EMD Serono, Novartis, and Roche/Genentech.
Figures





References
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372(9648): 1502–1517. - PubMed
-
- McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: A review. JAMA 2021; 325(8): 765–779. - PubMed
-
- Saccà F, Lanzillo R, Signori A, et al. Determinants of therapy switch in multiple sclerosis treatment-naïve patients: A real-life study. Mult Scler 2019; 25(9): 1263–1272. - PubMed
-
- Novartis Mayzent (siponimod). Prescribing information. Basel, Switzerland: Novartis, 2022.
-
- European Medicines Agency. Mayzent. Summary of product characteristics. Amsterdam, The Netherlands: European Medicines Agency, 2024.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

