Hypoxic Preconditioning Enhances the Potential of Mesenchymal Stem Cells to Treat Neonatal Hypoxic-Ischemic Brain Injury
- PMID: 40248869
- PMCID: PMC12180708
- DOI: 10.1161/STROKEAHA.124.048964
Hypoxic Preconditioning Enhances the Potential of Mesenchymal Stem Cells to Treat Neonatal Hypoxic-Ischemic Brain Injury
Abstract
Background: Neonatal hypoxic-ischemic (HI) brain injury is one of the leading causes of long-term neurological morbidity in newborns. Current treatment options for HI brain injury are limited, but mesenchymal stem cell (MSC) therapy is a promising strategy to boost neuroregeneration after injury. Optimization strategies to further enhance the potential of MSCs are under development. The current study aimed to test the potency of hypoxic preconditioning of MSCs to enhance the therapeutic efficacy in a mouse model of neonatal HI injury.
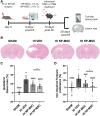
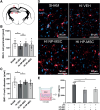
Methods: HI was induced on postnatal day 9 in C57Bl/6 mouse pups. MSCs were cultured under hypoxic (hypoxic-preconditioned MSCs [HP-MSCs], 1% O2) or normoxic-control (normoxic-preconditioned MSCs [NP-MSCs], 21% O2) conditions for 24 hours before use. At 10 days after HI, HP-MSCs, NP-MSCs, or vehicle were intranasally administered. Gold nanoparticle-labeled MSCs were used to assess MSC migration 24 hours after intranasal administration. At 28 days post-HI, lesion size, sensorimotor outcome, and neuroinflammation were assessed by hematoxylin and eosin staining, cylinder rearing task, and ionized calcium-binding adapter molecule 1 (IBA1) staining, respectively. In vitro, the effect of HP-MSCs was studied on transwell migration, neural stem cell differentiation and microglia activation, and the MSC intracellular proteomic content was profiled using quantitative Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS).
Results: Intranasally administered HP-MSCs were superior to NP-MSCs in reducing lesion size and sensorimotor impairments post-HI. Moreover, hypoxic preconditioning enhanced MSC migration in an in vitro set-up, and in vivo to the lesioned hemisphere after intranasal application. In addition, HP-MSCs enhanced neural stem cell differentiation into more complex neurons in vitro but had similar anti-inflammatory effects compared with NP-MSCs. Lastly, hypoxic preconditioning led to elevated abundances of proteins in MSCs related to extracellular matrix remodeling.
Conclusions: This study shows for the first time that hypoxic preconditioning enhanced the therapeutic efficacy of MSC therapy in a mouse model of neonatal HI brain injury by increasing the migratory and neuroregenerative capacity of MSCs.
Keywords: brain injuries; hypoxia-ischemia, brain; mesenchymal stem cells; neonate; neurogenesis; proteomics.
Conflict of interest statement
None.
Figures



References
-
- Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, Niermeyer S, Ellis M, Robertson NJ, Cousens S, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74:50–72. doi: 10.1038/pr.2013.206 - PMC - PubMed
-
- Perez A, Ritter S, Brotschi B, Werner H, Caflisch J, Martin E, Latal B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J Pediatr. 2013;163:454–459. doi: 10.1016/j.jpeds.2013.02.003 - PubMed
-
- Kariholu U, Montaldo P, Markati T, Lally P, Pryce R, Teiserskas J, Liow N, Oliveira V, Soe A, Shankaran S, et al. Therapeutic hypothermia for mild neonatal encephalopathy: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2020;105:F225–F228. doi: 10.1136/archdischild-2018-315711 - PubMed
-
- Baak LM, Wagenaar N, van der Aa NE, Groenendaal F, Dudink J, Tataranno ML, Mahamuud U, Verhage CH, Eijsermans RMJC, Smit LS, et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. Lancet Neurol. 2022;21:528–536. doi: 10.1016/S1474-4422(22)00117-X - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

