Diagnostic MicroRNA Signatures to Support Classification of Pulmonary Hypertension
- PMID: 40248872
- PMCID: PMC12173163
- DOI: 10.1161/CIRCGEN.124.004862
Diagnostic MicroRNA Signatures to Support Classification of Pulmonary Hypertension
Abstract
Background: Patients with pulmonary hypertension (PH) are classified based on disease pathogenesis and hemodynamic drivers. Classification informs treatment. The heart failure biomarker NT-proBNP (N-terminal pro-B-type natriuretic peptide) is used to help inform risk but is not specific to PH or sub-classification groups. There are currently no other biomarkers in clinical use to help guide diagnosis or risk.
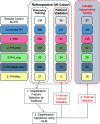
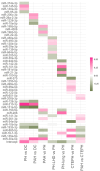
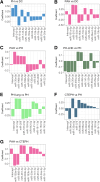
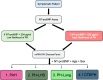
Methods: We profiled a retrospective cohort of 1150 patients from 3 expert centers with PH and 334 non-PH symptomatic controls (disease controls) from the United Kingdom to measure circulating levels of 650 microRNAs (miRNAs) in serum. NT-proBNP (ELISA) and 326 well-detected miRNAs (polymerase chain reaction) were prioritized by feature selection using multiple machine learning models. From the selected miRNAs, generalized linear models were used to describe miRNA signatures to differentiate PH and pulmonary arterial hypertension from the disease controls, and pulmonary arterial hypertension, PH due to left heart disease, PH due to lung disease, and chronic thromboembolic pulmonary hypertension from other forms of PH. These signatures were validated on a UK test cohort and independently validated in the prospective CIPHER study (A Prospective, Multicenter, Noninterventional Study for the Identification of Biomarker Signatures for the Early Detection of Pulmonary Hypertension) comprising 349 patients with PH and 93 disease controls.
Results: NT-proBNP achieved a balanced accuracy of 0.74 and 0.75 at identifying PH and pulmonary arterial hypertension from disease controls with a threshold of 254 and 362 pg/mL, respectively but was unable to sub-categorize PH subgroups. In the UK cohort, miRNA signatures performed similarly to NT-proBNP in distinguishing PH (area under the curve of 0.7 versus 0.78), and pulmonary arterial hypertension (area under the curve of 0.73 versus 0.79) from disease controls. MicroRNA signatures outperformed NT-proBNP in distinguishing PH classification groups. External testing in the CIPHER cohort demonstrated that miRNA signatures, in conjunction with NT-proBNP, age, and sex, performed better than either NT-proBNP or miRNAs alone in sub-classifying PH.
Conclusions: We suggest a threshold for NT-proBNP to identify patients with a high probability of PH, and the subsequent use of circulating miRNA signatures to help differentiate PH subgroups.
Keywords: biomarkers; early diagnosis; machine learning; miRNA; pulmonary hypertension.
Conflict of interest statement
Drs Wilkins, Lawrie, Wang, Rhodes, Errington, Toshner, Fong, Jatkoe, He, and Lihan are named inventors in pending patent applications directed to aspects of this article. Drs Fong and Jatkoe were employees of Janssen Pharmaceutical Companies of Johnson & Johnson and own shares of stock/stock options in Johnson & Johnson. The other authors report no conflicts.
Figures


Similar articles
-
Brain natriuretic peptide and N-terminal brain natriuretic peptide for the diagnosis of haemodynamically significant patent ductus arteriosus in preterm neonates.Cochrane Database Syst Rev. 2022 Dec 8;12(12):CD013129. doi: 10.1002/14651858.CD013129.pub2. Cochrane Database Syst Rev. 2022. PMID: 36478359 Free PMC article.
-
Predictive Value of Smoking Index Combined with NT-proBNP for Patients with Pulmonary Hypertension Due to Chronic Lung Disease: A Retrospective Study.Int J Chron Obstruct Pulmon Dis. 2024 Jun 4;19:1233-1245. doi: 10.2147/COPD.S448496. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38854590 Free PMC article.
-
B-type natriuretic peptide as a parameter for pulmonary hypertension in children. A systematic review.Eur J Pediatr. 2015 Oct;174(10):1267-75. doi: 10.1007/s00431-015-2619-0. Epub 2015 Aug 23. Eur J Pediatr. 2015. PMID: 26298682
-
The comparative and added prognostic value of biomarkers to the Revised Cardiac Risk Index for preoperative prediction of major adverse cardiac events and all-cause mortality in patients who undergo noncardiac surgery.Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD013139. doi: 10.1002/14651858.CD013139.pub2. Cochrane Database Syst Rev. 2021. PMID: 34931303 Free PMC article.
-
Evidence-Based Application of Natriuretic Peptides in the Evaluation of Chronic Heart Failure With Preserved Ejection Fraction in the Ambulatory Outpatient Setting.Circulation. 2025 Apr 8;151(14):976-989. doi: 10.1161/CIRCULATIONAHA.124.072156. Epub 2025 Jan 22. Circulation. 2025. PMID: 39840432
Cited by
-
Editorial: Exploring the role of epigenetic modifications in pulmonary vascular disease pathogenesis.Front Med (Lausanne). 2025 May 16;12:1618278. doi: 10.3389/fmed.2025.1618278. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40454143 Free PMC article. No abstract available.
References
-
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, et al. ; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61:2200879. doi: 10.1183/13993003.00879-2022 - PubMed
-
- Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, Joynt KE, Kass DJ, Stephens T, Stanislawski MA, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207 - PMC - PubMed
-
- Khou V, Anderson JJ, Strange G, Corrigan C, Collins N, Celermajer DS, Dwyer N, Feenstra J, Horrigan M, Keating D, et al. Diagnostic delay in pulmonary arterial hypertension: insights from the Australian and New Zealand pulmonary hypertension registry. Respirology. 2020;25:863–871. doi: 10.1111/resp.13768 - PubMed
-
- Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612–622. doi: 10.1136/hrt.2010.212084 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

