Ca2+ Cycling Alteration in a Porcine Model of Right Ventricular Dysfunction
- PMID: 40248873
- PMCID: PMC12084022
- DOI: 10.1161/CIRCHEARTFAILURE.124.012293
Ca2+ Cycling Alteration in a Porcine Model of Right Ventricular Dysfunction
Abstract
Background: Pulmonary hypertension is a severe disease with high mortality rates due to right ventricular (RV) failure. The molecular and cellular processes involved in RV remodeling, including Ca2+ handling, remain elusive due to the lack of relevant animal models. In this study, we aim to understand better the pathophysiological mechanisms involved in RV failure.
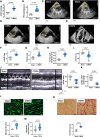
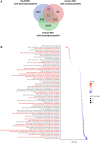
Methods: We used the chronic thromboembolic pulmonary hypertension (CTEPH) pig model, which leads to progressive RV hypertrophy and dysfunction. Cellular, molecular unbiased global transcriptional profiling and biochemical analyses were performed on RV cardiomyocytes from CTEPH and Sham-operated pigs.
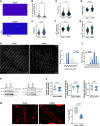
Results: CTEPH pigs replicated the hemodynamics and histological changes of human CTEPH features. Transcriptome analysis in Sham and CTEPH pigs revealed molecular RV remodeling close to human patients with pulmonary arterial hypertension with decompensated RV function and notably identified changes in genes involved in Ca2+ signaling. At the cellular level, CTEPH myocytes presented reduced L-type Ca2+ current in association with reduced mRNA of CACNA1C. Furthermore, CTEPH myocytes showed lower [Ca2+]i transients, decreased sarcoplasmic reticulum Ca2+ content, and decreased cell shortening, related to reduced SERCA2a (Sarco/endoplasmic reticulum Ca2+-ATPase isoform 2a) protein expression. Moreover, CTEPH cardiomyocytes exhibited reduced Ca2+ spark occurrence, which relied on smaller RyR2 (ryanodine receptor 2) clusters and T-tubule disorganization. Finally, these alterations in Ca2+ homeostasis were also associated with an increased store-operated Ca2+ entry and the de novo expression of the Ca2+ sensor protein STIM1L (stromal interaction molecule 1 long isoform) in CTEPH myocytes as well as in RV from human patients with pulmonary arterial hypertension.
Conclusions: Our data reveal cellular Ca2+ cycling remodeling that participates in the pathogenesis of RV dysfunction and may constitute therapeutic targets to limit the development of RV dysfunction.
Keywords: calcium signaling; excitation contraction coupling; humans; pulmonary hypertension; right-sided heart failure; swine.
Conflict of interest statement
Dr Humbert has relationships with drug companies, including Acceleron, AOP Orphan, Merck, Chiesi, Ferrer, Janssen, MorphogenIX, Shou Ti, Tiakis, and United Therapeutics. Dr Montani has relationships with drug companies, including Actelion, Bayer, GSK, Novartis, and Pfizer. Dr Jaïs has relationships with drug companies, including Janssen, Bayer, and Merck. In addition to being investigators in trials involving these companies, other relationships include consultancy services and memberships to scientific advisory boards. The other authors report no conflicts.
Figures





References
-
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, et al. ; ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61:2200879. doi: 10.1183/13993003.00879-2022 - PubMed
-
- Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53:1801900. doi: 10.1183/13993003.01900-2018 - PMC - PubMed
-
- Andersen S, Nielsen-Kudsk JE, Vonk Noordegraaf A, de Man FS. Right ventricular fibrosis. Circulation. 2019;139:269–285. doi: 10.1161/CIRCULATIONAHA.118.035326 - PubMed
-
- Llucià-Valldeperas A, de Man FS, Bogaard HJ. Adaptation and maladaptation of the right ventricle in pulmonary vascular diseases. Clin Chest Med. 2021;42:179–194. doi: 10.1016/j.ccm.2020.11.010 - PubMed
-
- Humbert M, Lau EMT, Montani D, Jaïs X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014;130:2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

