Innate immune sensor NLRP3 drives PANoptosome formation and PANoptosis
- PMID: 40249072
- PMCID: PMC12207079
- DOI: 10.1093/jimmun/vkaf042
Innate immune sensor NLRP3 drives PANoptosome formation and PANoptosis
Abstract
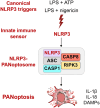
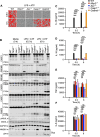
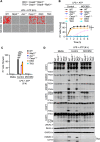
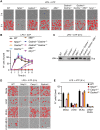
Inflammasomes are multiprotein innate immune complexes formed in response to infections, tissue damage, or cellular stress that promote the maturation and release of IL-1β/IL-18 and are implicated in lytic cell death. The NLRP3 inflammasome is canonically activated by an initial priming event followed by an activation stimulus, leading to rapid cell death that occurs through caspase-1 (CASP1) and gasdermin D (GSDMD) activation, called pyroptosis. CASP1- and GSDMD-deficient cells are protected from the rapid LPS plus ATP-induced pyroptosis. However, innate immune responses physiologically occur over time, extending beyond minutes to hours and days. Therefore, in this study, we assessed lytic cell death beyond the early timepoints. While cells lacking the innate immune sensor NLRP3 were protected from cell death induced by the canonical NLRP3 trigger, LPS priming and ATP stimulation (LPS plus ATP), for extended time, CASP1- and GSDMD-deficient cells started to lyse in a time-dependent manner after 2 h. Nevertheless, robust IL-1β and IL-18 release was still dependent on CASP1 activation. These data suggested that NLRP3 engages an additional innate immune, lytic cell death pathway. Indeed, LPS plus ATP induced the activation of caspases and RIPKs associated with PANoptosis in WT cells, and cells deficient in PANoptosis machinery were protected from cell death for extended times. A PANoptosome complex containing NLRP3, ASC, CASP8, and RIPK3 was observed by microscopy in WT, as well as CASP1- or GSDMD-deficient, cells by 30 min post-stimulation. Overall, these findings highlight the central role of NLRP3 as a PANoptosome sensor. Given the physiological role of innate immune cell death, PANoptosis, in health and disease, our study emphasizes the importance of a comprehensive understanding of PANoptosomes, and their components, as therapeutic targets.
Keywords: PANoptosome; RIPK; caspase; inflammasome; inflammation.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
T.-D.K. was a consultant for Pfizer.
Figures
References
-
- Kanneganti TD et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. - PubMed
-
- Franchi L et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

