Antitumor CD4+ T Helper 1 Cells Target and Control the Outgrowth of Disseminated Cancer Cells
- PMID: 40249209
- PMCID: PMC12046335
- DOI: 10.1158/2326-6066.CIR-24-0630
Antitumor CD4+ T Helper 1 Cells Target and Control the Outgrowth of Disseminated Cancer Cells
Abstract
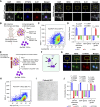
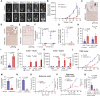
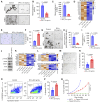
Detection of disseminated cancer cells (DCC) in the bone marrow (BM) of patients with breast cancer is a critical predictor of late recurrence and distant metastasis. Conventional therapies often fail to completely eradicate DCCs in patients. In this study, we demonstrate that intratumoral priming of antitumor CD4+ T helper 1 (Th1) cells was able to eliminate the DCC burden in distant organs and prevent overt metastasis, independent of CD8+ T cells. Intratumoral priming of tumor antigen-specific CD4+ Th1 cells enhanced their migration to the BM and distant metastatic site to selectively target DCC burden. The majority of these intratumorally activated CD4+ T cells were CD4+PD1- T cells, supporting their nonexhaustion stage. Phenotypic characterization revealed enhanced infiltration of memory CD4+ T cells and effector CD4+ T cells in the primary tumor, tumor-draining lymph node, and DCC-driven metastasis site. A robust migration of CD4+CCR7+CXCR3+ Th1 cells and CD4+CCR7-CXCR3+ Th1 cells into distant organs further revealed their potential role in eradicating DCC-driven metastasis. The intratumoral priming of antitumor CD4+ Th1 cells failed to eradicate DCC-driven metastasis in CD4- or IFN-γ knockout mice. Moreover, antitumor CD4+ Th1 cells, by increasing IFN-γ production, inhibited various molecular aspects and increased classical and nonclassical MHC molecule expression in DCCs. This reduced stemness and self-renewal while increasing immune recognition in DCCs of patients with breast cancer. These results unveil an immune basis for antitumor CD4+ Th1 cells that modulate DCC tumorigenesis to prevent recurrence and metastasis in patients.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
G. Ramamoorthi reports a patent for PCT/US2024/013114 pending to Moffitt Cancer Center. M.C. Lee reports other support from private donors during the conduct of the study. B.J. Czerniecki reports grants from CDMRP/DOD, Pennies in Action, and CDMRP/DOD during the conduct of the study as well as other support from ImmunoRestoration outside the submitted work and has a patent for use of syringe ready type I dendritic cells pending. No disclosures were reported by the other authors.
Figures




References
-
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. . A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005;353:793–802. - PubMed
-
- Hartkopf AD, Brucker SY, Taran FA, Harbeck N, von Au A, Naume B, et al. . Disseminated tumour cells from the bone marrow of early breast cancer patients: results from an international pooled analysis. Eur J Cancer 2021;154:128–37. - PubMed
-
- Hartkopf AD, Wallwiener M, Fehm TN, Hahn M, Walter CB, Gruber I, et al. . Disseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapse. Ann Oncol 2015;26:1155–60. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

