Phosphatidylinositol 5-Phosphate-Loaded Apoptotic Body-Like Liposomes for Mycobacterium abscessus Infection Management in Patients With Cystic Fibrosis
- PMID: 40249250
- PMCID: PMC12308664
- DOI: 10.1093/infdis/jiaf124
Phosphatidylinositol 5-Phosphate-Loaded Apoptotic Body-Like Liposomes for Mycobacterium abscessus Infection Management in Patients With Cystic Fibrosis
Abstract
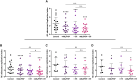
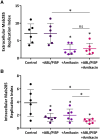
The study investigates therapeutic strategies for managing chronic Mycobacterium abscessus infections, particularly in people with cystic fibrosis (PWCF) who are ineligible for standard elexacaftor, tezacaftor, ivakaftor (ETI) treatments. Apoptotic body-like liposomes loaded with phosphatidylinositol 5-phosphate (ABL/PI5P) were tested in vitro in M. abscessus-infected macrophages from PWCF as potential treatment. ABL/PI5P reduced intracellular bacterial viability and showed enhanced effects on a M. abscessus clinical strain when combined with amikacin. Notably, ABL/PI5P was effective on macrophages from PWCF not receiving ETI therapy. The findings suggest ABL/PI5P liposomes as a promising alternative or adjunct therapy, especially for those who cannot access ETI treatment, warranting further clinical investigation.
Keywords: Mycobacterium abscessus; ETI; cystic fibrosis; host-directed therapy; innate immunity; liposomes; pathogen-directed therapy; phosphatidylinositol 5-phosphate.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. F., T. O., and N. P. are cofounders of Bioactive Liposome Therapeutics, Srls, spin-off of Tor Vergata University of Rome, focusing on the development of novel bioactive liposome-based host-directed therapies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

