Operando Measurement of Transition Metal Deposition in a NMC Li-Ion Battery Using Laboratory Confocal Micro-X-ray Fluorescence Spectroscopy
- PMID: 40249459
- PMCID: PMC12177848
- DOI: 10.1002/smll.202502460
Operando Measurement of Transition Metal Deposition in a NMC Li-Ion Battery Using Laboratory Confocal Micro-X-ray Fluorescence Spectroscopy
Abstract
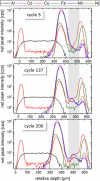
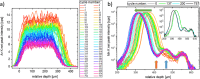
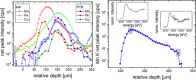
The degradation of batteries has very different causes depending on the material and operation modes. However, most of these causes are associated with changes in one or more interfaces, in particular through depositions and their potential chemical changes under operating conditions. Over the last decade operando investigations have therefore become increasingly state-of-the-art, elemental analysis of full cell systems, though, is still missing due to a lack of depth resolved methods. Using laboratory confocal micro-X-ray fluorescence spectroscopy the analysis of a Li-ion battery coin cell during 10600 cycles are presented. It is shown that the confocal setup enables to differentiate between the nickel-manganese-cobalt-oxide (NMC) cathode with high levels of transition metal concentration and a possible deposition of traces of Mn, Ni, Co in the underlying layers. This allows for spatially resolved insights in operando without changing the layer stack, nor electrode area. This paper is the first to demonstrate the non-destructive and quantitative elemental analysis of battery interfaces under operating conditions. This quantitative analysis is the prerequisite for the determination of absolute transport and conversion rates, without which the transition from empirical research to a focused development of batteries will not succeed.
Keywords: CR2032 coin cell; NMC Li ion batteries; confocal micro X‐ray fluorescence; non‐destructive testing; operando elemental investigations.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Tarascon J.‐M., Armand M., Nature 2001, 414, 359. - PubMed
-
- Jiang X., Ma J., Xiao G., Shao Z., Guo X., Inf. Fusion 2021, 73, 22.
-
- Li T., Yuan X.‐Z., Zhang L., Song D., Shi K., Bock C., Electrochem. Energy Rev. 2020, 3, 43.
-
- Jung R., Metzger M., Maglia F., Stinner C., Gasteiger H. A., J. Electrochem. Soc. 2017, 164, A1361 . - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

